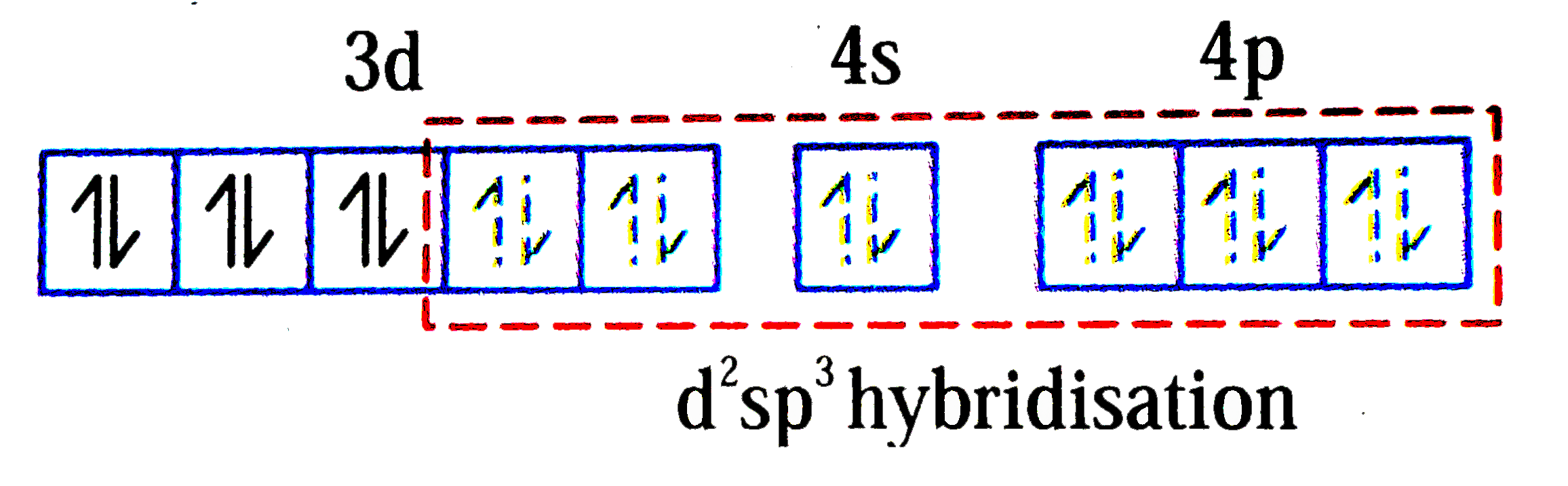A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPLEXES
NARAYNA|Exercise EXERCISE-6|6 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise EXERCISE-7|5 VideosCOORDINATION COMPLEXES
NARAYNA|Exercise Additional questions page-155|10 VideosCO-ORDINATE COMPOUNDS
NARAYNA|Exercise Exercise-4|60 VideosD - BLOCK ELEMENTS
NARAYNA|Exercise CHECK YOUR GRASP|7 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-COORDINATION COMPLEXES-EXERCISE-5
- A complex cation is formed by Pt (in some oxidation state) with ligand...
Text Solution
|
- The correct order of their paramagnetic moment (spin only) is P:[FeF(6...
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- What will be the 'spin only' magnetic moment of the complex formed whe...
Text Solution
|
- The compound having a tetrahedral geometry is .
Text Solution
|
- It is an experimental fact that Cs(2)[CuCl(4)] is orange coloured but ...
Text Solution
|
- The hybridization of the d-block element sodium nitropruside is
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Amongst [Co(o x)(3)]^(3-),[CoF(6)]^(3-) and [Co(NH(3))(6)]^(3+) :
Text Solution
|
- All the following complexes show decrease in their weights when placed...
Text Solution
|
- The geometry of [NiCl(4)]^(2-) and [Ni(PPh(3))(2)Cl(2)]are :
Text Solution
|
- [Co(en)(2)(H(2)O)(2)]^(3+)+en to complex (X) is :
Text Solution
|
- Which one of the following complexes exhibit chirality?
Text Solution
|
- On treatment of [Pt(NH(3))(4)]^(2+ with Cl compound I is formed When [...
Text Solution
|
- For the empirical formula, Pt(NH(3))(2)Cl(2) the number of possiblecoo...
Text Solution
|
- The total number of isomer shown by [Co(NH(3))(4)(NO(2))(2)](NO(3)) co...
Text Solution
|