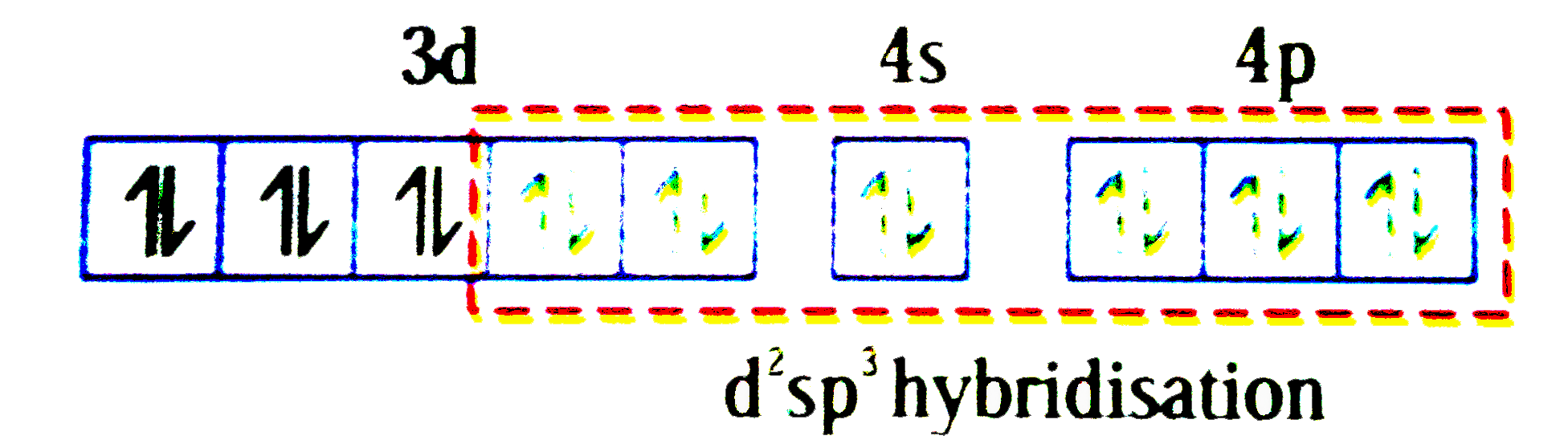
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-COORDINATION COMPLEXES-EXERCISE-7
- Which one of the following has the correct order of Delta(o) ?
Text Solution
|
- Wilkinson's catalyst react with H(2) to from an octahedral complex in ...
Text Solution
|
- In the cystal field of the complex [Fe(Cl)(CN)(4)(O(2))]^(4-) the elec...
Text Solution
|
- Select the correct statement (s) for the coordination compound K(2)...
Text Solution
|
- Which of the following complex (s) is/are correctly matched with their...
Text Solution
|