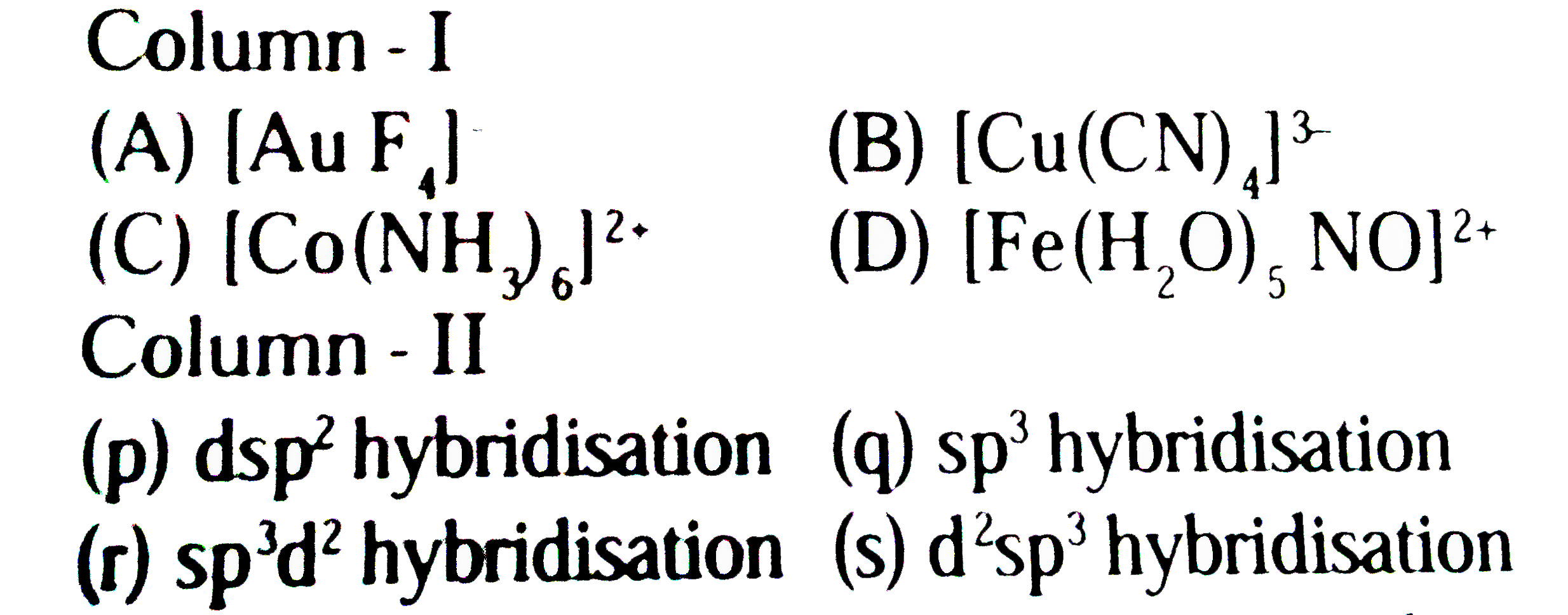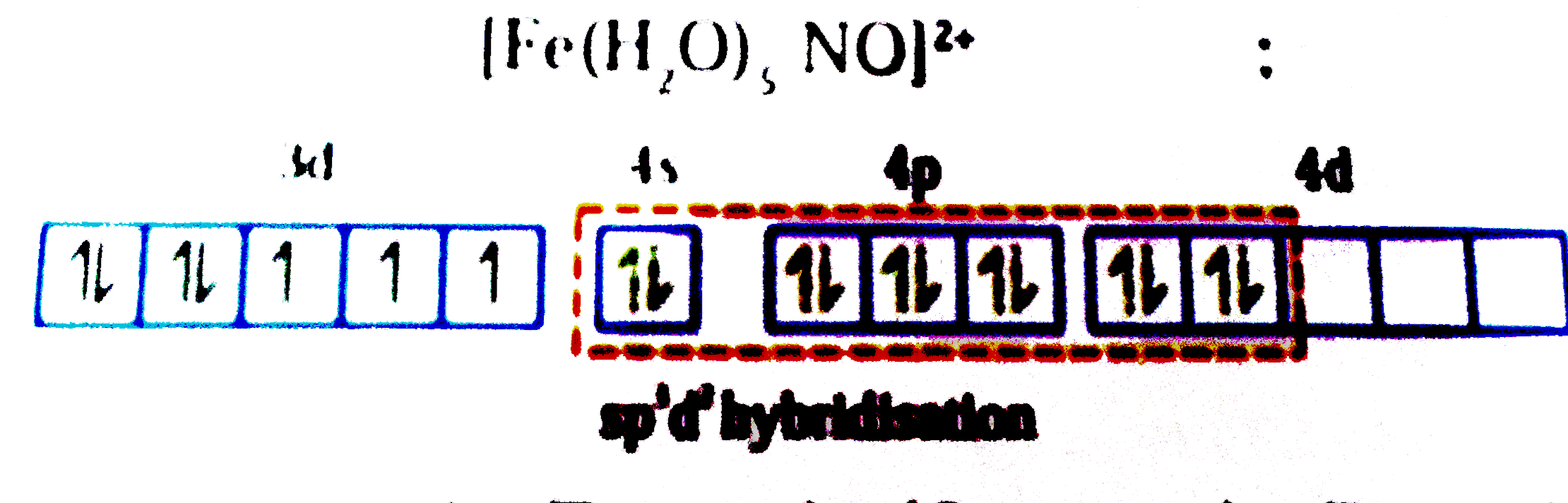Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-COORDINATION COMPLEXES-EXERCISE-8
- Match the complexes listed in column-I with characteristic (s)/type of...
Text Solution
|
- Match the complexes listed in column-I with characteristic(s) listed i...
Text Solution
|
- Match the complexes listed in column-I with characteristic (s)/type hy...
Text Solution
|
- Match the complexes listed in column-I with characteristic (s)/type of...
Text Solution
|
- Match the complexes listed in colmn-I with type hybridisation listed i...
Text Solution
|
- Select the correct option (s) for the coordination compounds and their...
Text Solution
|