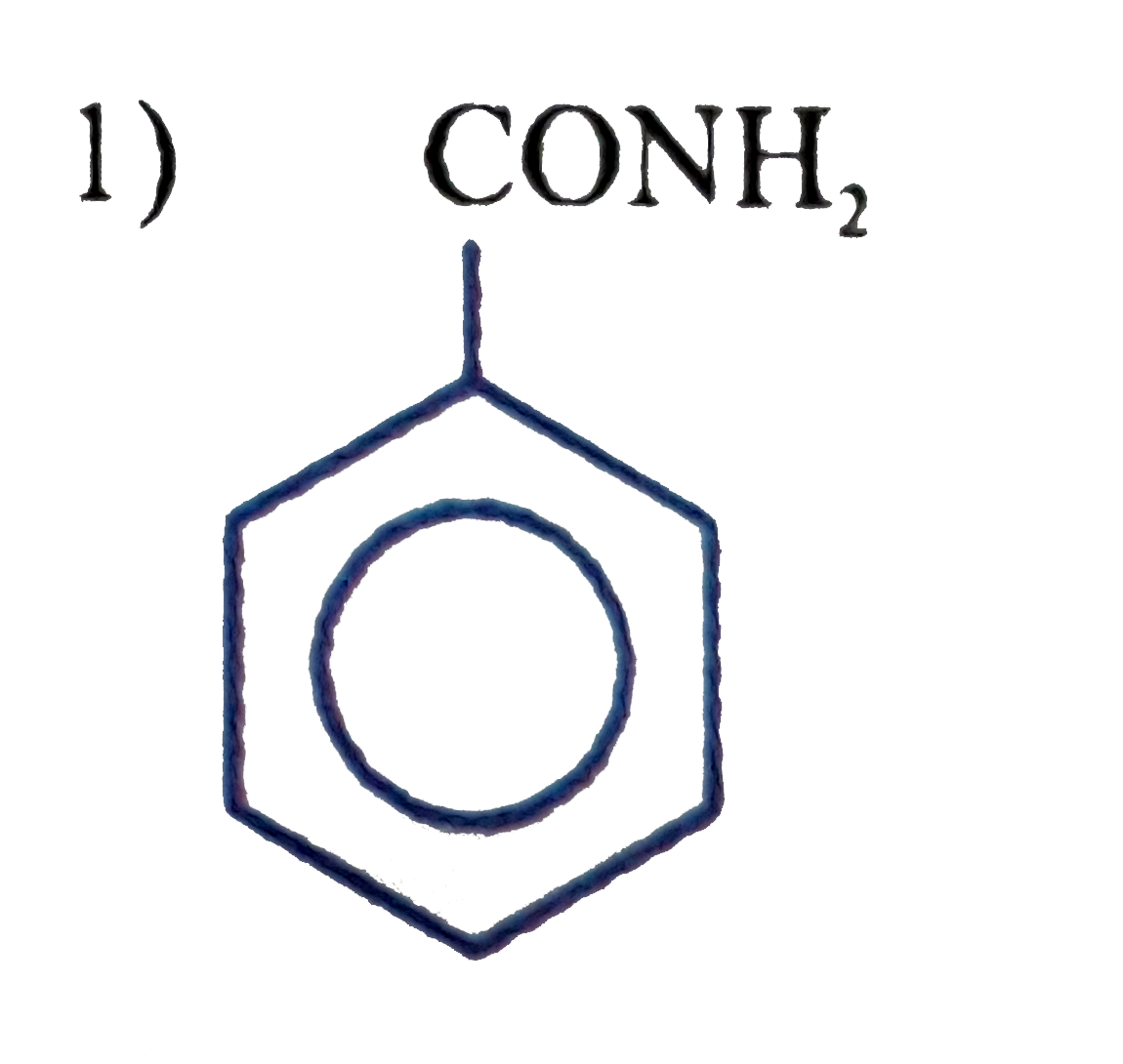
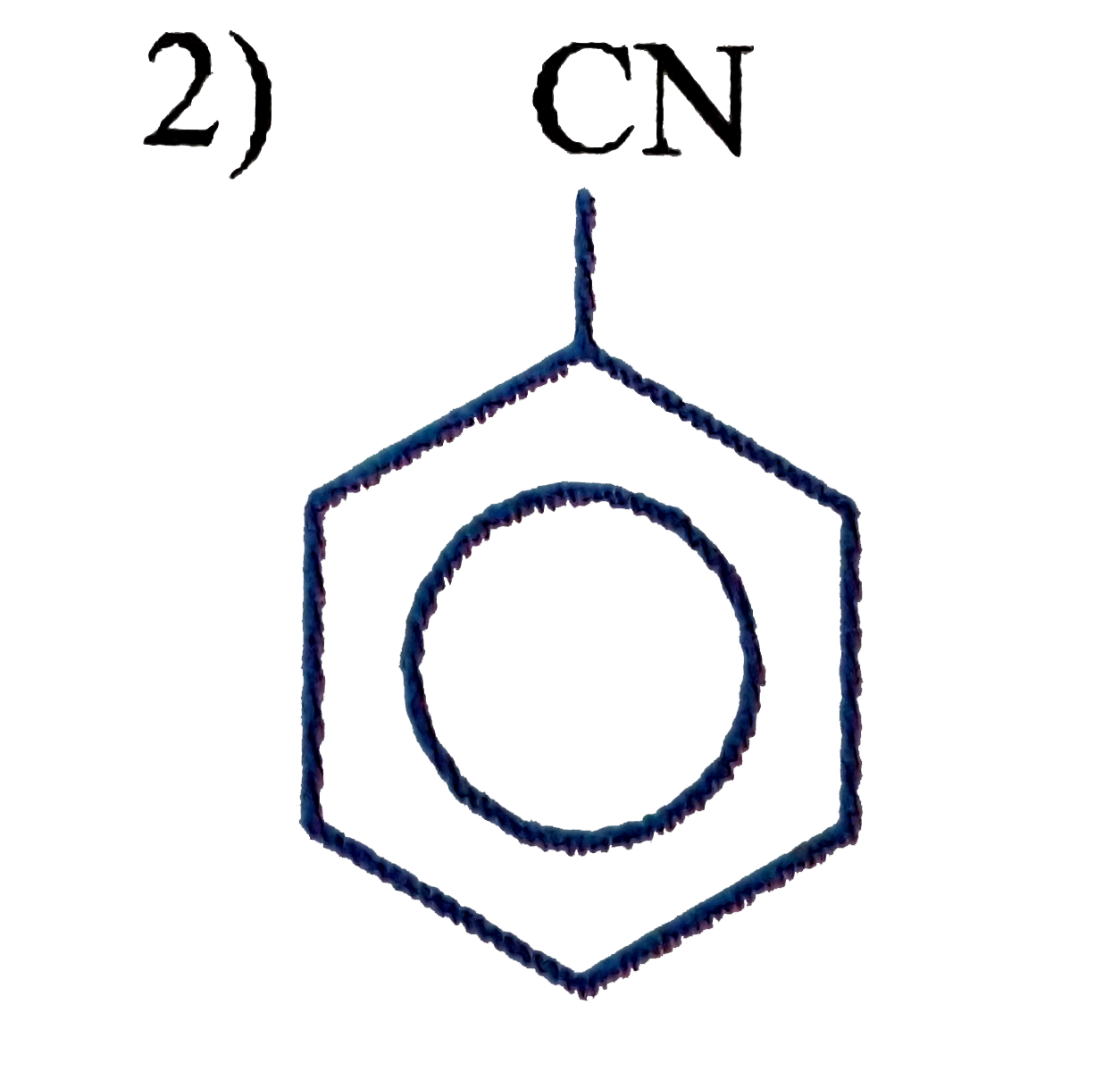
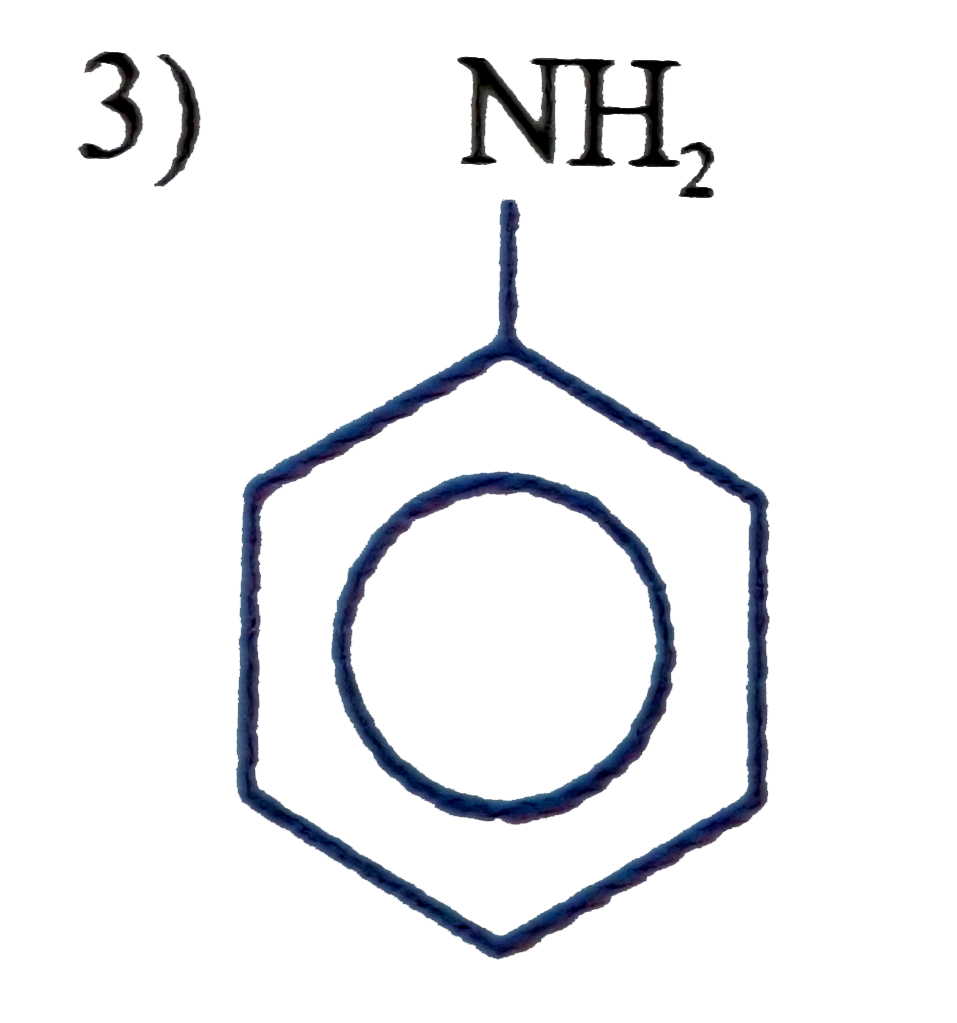
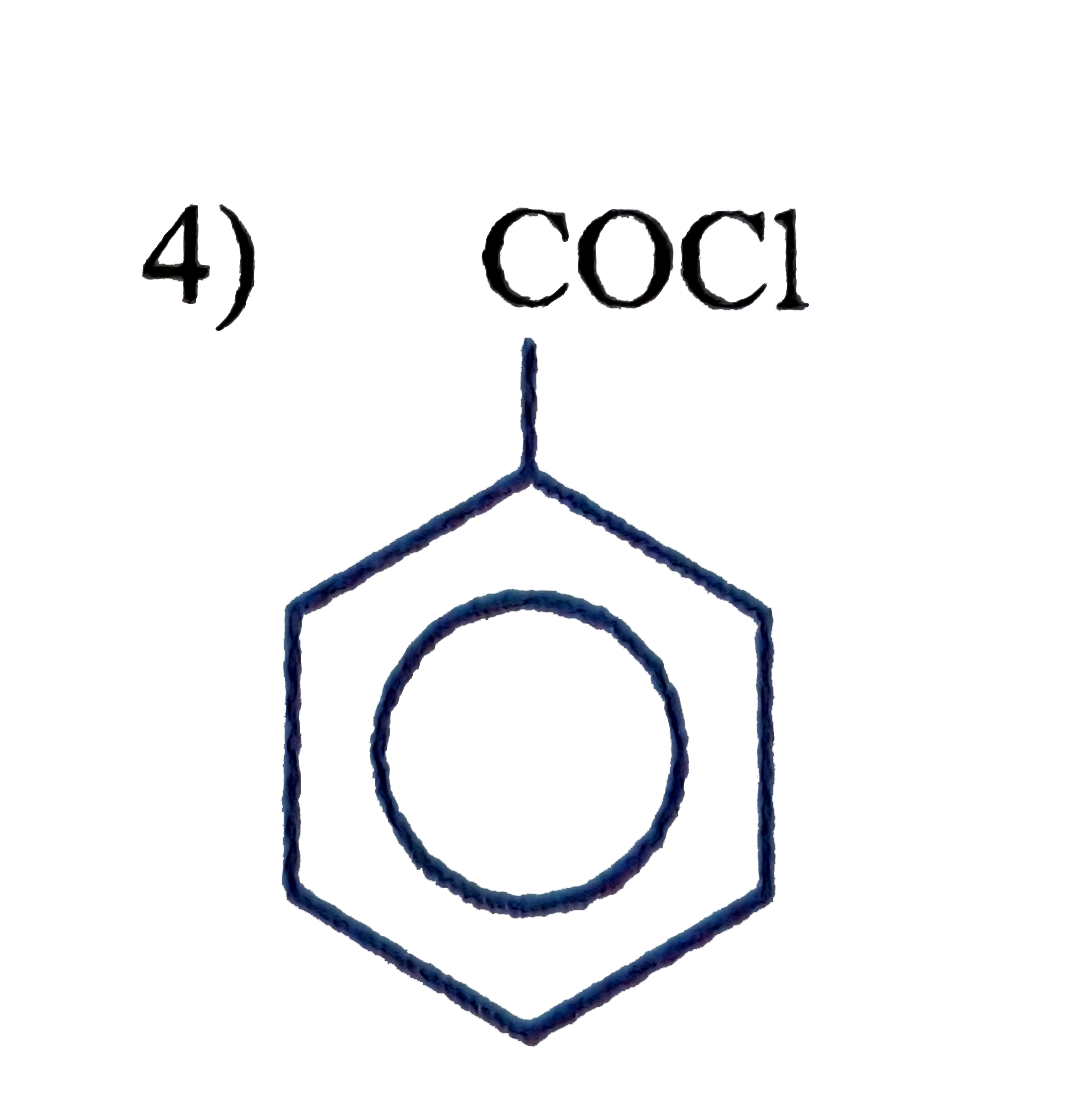
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level -I (H.w)|19 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level II (H.W.)|15 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level -III|19 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise CHECK YOUR GRASP|6 VideosBIOMOLECULES
NARAYNA|Exercise EXERCISE-4 (NCERT EXAMPLAR PROBLEMS)|12 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-AMINES & DIAZO COMPOUNDS-Level -IV
- Electron releasing group pushes electrons to ward nitrogen and hence i...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- An aromatic compound 'A' on treatment with aqueous ammonia and heating...
Text Solution
|
- Which of the following is a 3^(@) amine
Text Solution
|
- The correct IUPAC name for CH2 ==CH CH2 NH CH3 is
Text Solution
|
- Which of the following reagents would not be a good choice for reducin...
Text Solution
|
- In order to prepare a 1^(@) amine from an alkyl halide with simultaneo...
Text Solution
|
- The source of nitrogen in Gabriel syntheisis of amine is..
Text Solution
|
- Amongst the given set of reactants, the most approprate for preparing ...
Text Solution
|
- The best reagent for converting 2-phenylpropanamide into 2-phenylpropa...
Text Solution
|
- Hofmann's bromamide degradation reaction is shown by
Text Solution
|
- Which of the following is a 3^(@) amine
Text Solution
|
- Amongst the following, the strongest base in aqueous medium is
Text Solution
|
- Which of the following is the weakest Bronsted base?
Text Solution
|
- Benzylamine may be alkylated as shown in the following equation C(...
Text Solution
|
- The correct increasing order of basic strength of the following compou...
Text Solution
|
- Methylamine reacts with HNO(2) to form….
Text Solution
|
- The gas evolved when methylamine reacts with nitrous acid is….
Text Solution
|
- In the nitration of benzene using a mixture of conc. H(2)SO(4) and con...
Text Solution
|