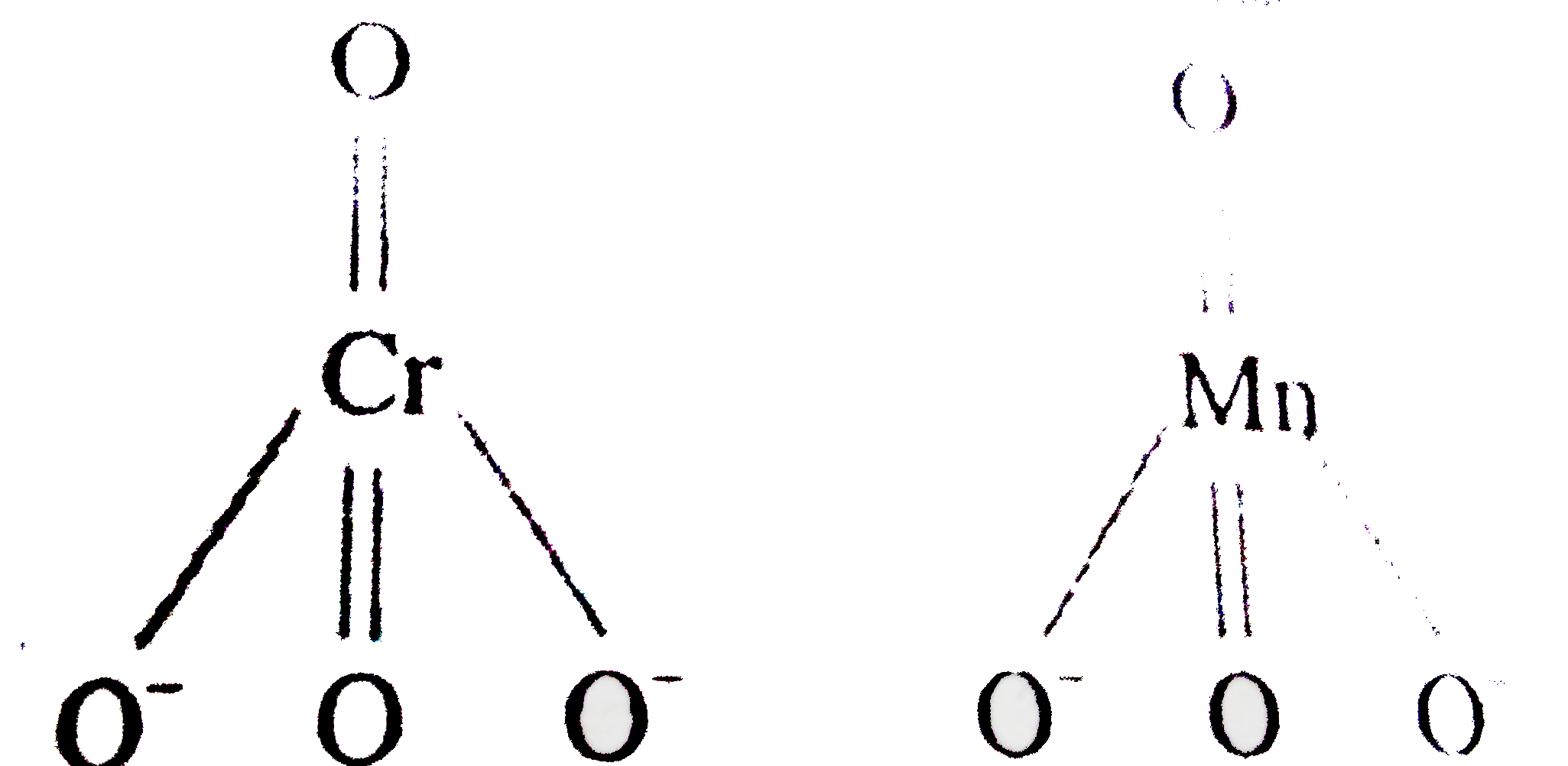
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY OF HEAVY METALS
NARAYNA|Exercise Level IV NCERT BASED QUESTIONS|22 VideosCHEMISTRY OF HEAVY METALS
NARAYNA|Exercise Level-V multiple answer questions|27 VideosCHEMISTRY OF HEAVY METALS
NARAYNA|Exercise SINGle Answer Questions(LEVEL-II)|36 VideosCHEMISTRY IN EVERYDAY LIFE
NARAYNA|Exercise ASSERTION & REASON TYPE QUESTIONS|10 VideosCO-ORDINATE COMPOUNDS
NARAYNA|Exercise Exercise-4|60 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-CHEMISTRY OF HEAVY METALS-Level III Single Answer Questions
- Which of the following statement(s) is correct ?
Text Solution
|
- Copper sulphate is prepared by blowing a current of air through copper...
Text Solution
|
- Identify the wrong statement regarding copper sulphate :
Text Solution
|
- A white percipitate of AgCl dissolves in excess of I) NH(3)(aq)" " ...
Text Solution
|
- The oxoanion which contains all equivalent M-O bond is I) CrO(4)^(...
Text Solution
|
- Among the following which is most stable ? (1) underset((X))([Fe(CN)...
Text Solution
|
- Identify"C" in the following sequence FeCO(3)underset("in air")overs...
Text Solution
|
- A metal M and its compound can give the following observable changes i...
Text Solution
|
- When Cu powder is heated with CuCl(2) solution, the compound obtained ...
Text Solution
|
- Copper (II) is estimated by the addition of the following reagent
Text Solution
|
- Mohrs salt is used as a Primary standard in the permanganometric titra...
Text Solution
|
- (T) imparts violet colour overset(compd(U)+conc.H(2)SO(4))(to)(V)"Red ...
Text Solution
|
- MnO(4)^(-)+xe^(-)rarrMnO(4)^(2-) x,y and z are respectively
Text Solution
|