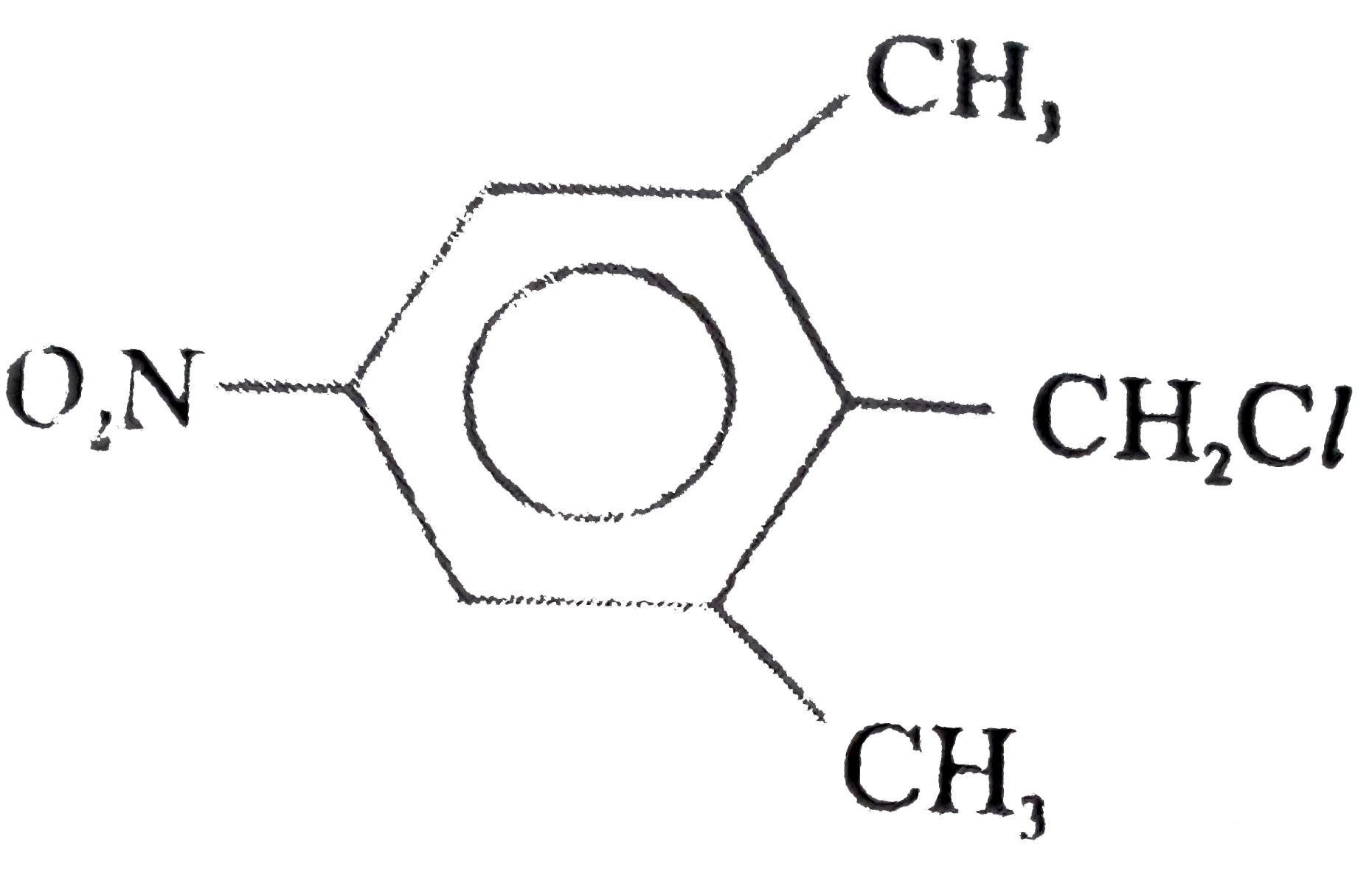
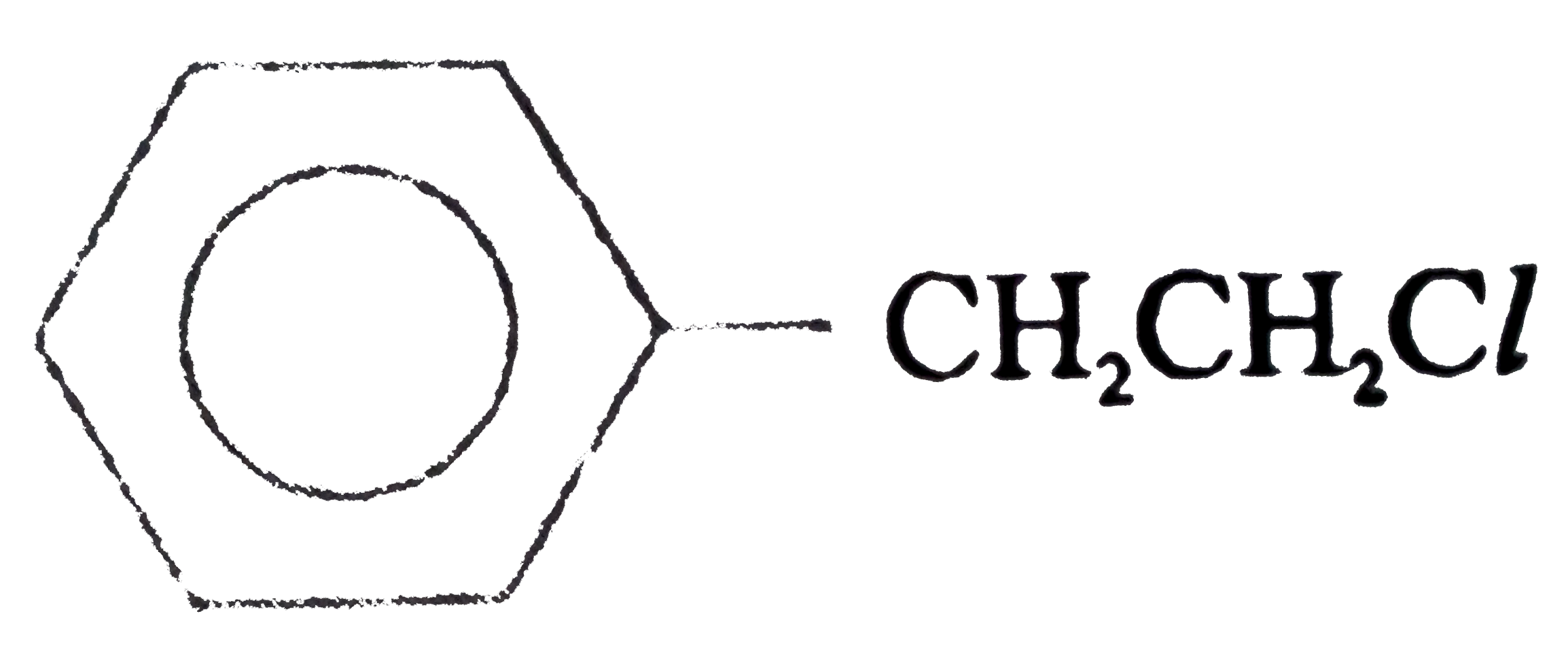
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PRACTICAL ORGANIC CHEMISTRY
NARAYNA|Exercise COMPREHENSION_TYPE|18 VideosPRACTICAL ORGANIC CHEMISTRY
NARAYNA|Exercise SUBJECTIVE_TYPE|1 VideosPRACTICAL ORGANIC CHEMISTRY
NARAYNA|Exercise MCQ_TYPE|30 VideosPOLYMERS
NARAYNA|Exercise EXERCISE -4|8 VideosQUALITATIVE ANALYSIS
NARAYNA|Exercise Subjective Type Question|33 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-PRACTICAL ORGANIC CHEMISTRY-SCQ_TYPE
- Sodium extract is heated with con. HNO(3) before testing for halogens ...
Text Solution
|
- To determinent the weight of a halogen in orangic compound, the compou...
Text Solution
|
- Detection of the chlorine is possible without preparing sodium extract...
Text Solution
|
- During the testing for phosphorus in organic compounds, a yellow solut...
Text Solution
|
- In Lassaigne’s test, the organic compound is fused with a piece of sod...
Text Solution
|
- In the Lassaigne's test for the detection of nitrogen in an organic co...
Text Solution
|
- In the Lassaigne’s test, the blood red colouration is due to the forma...
Text Solution
|
- In Lassaigne's test for nitrogen the blue colour is due to the formati...
Text Solution
|
- In Kjeldahl's method, nitrogen present is estimated as :
Text Solution
|
- The percentate of sulphur in the organic, when 0.2595g of a sulphur co...
Text Solution
|
- If 0.228 g of silver salt of dibasic acid gabe a residue of 0.162 g of...
Text Solution
|
- In Carius tube the compound ClCH(2)-COOH was heated with fuming HNO...
Text Solution
|
- Lassaigne's test for the detection of nitrogen fails in
Text Solution
|
- The compound that does not give a blue colour in Lassaigne's test is
Text Solution
|
- The Lassaigne's extract is boiled with dil. HNO(3) before testing for ...
Text Solution
|
- In Carius tube the compound ClCH(2)-COOH was heated with fuming HNO...
Text Solution
|
- If 0.24 g of a volatile liquid upon vaporization gives 45 ml of vapour...
Text Solution
|
- In sodium fusion test of roganilc compound, the nitrogen of a organic ...
Text Solution
|
- Which of the following will give blood red colour with FeCl(5) in sodi...
Text Solution
|
- Sulphide ions react with Na(2) [Fe(NO)(CN)(5)] to form a purple colour...
Text Solution
|