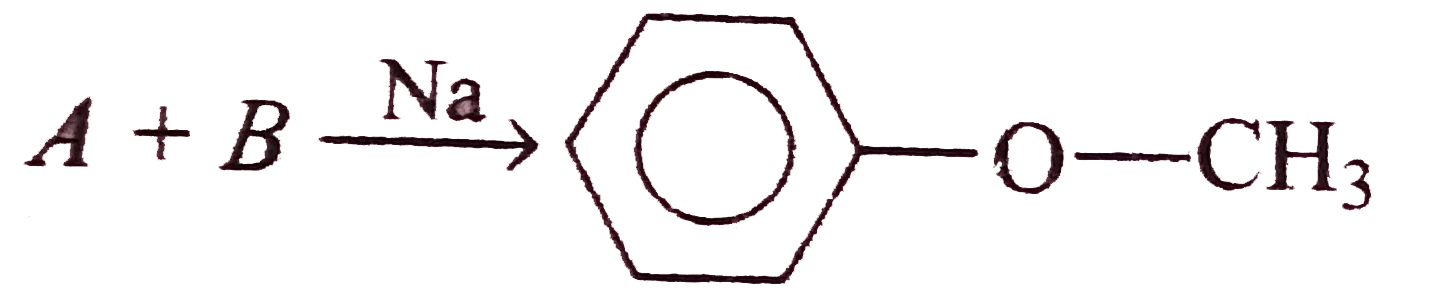
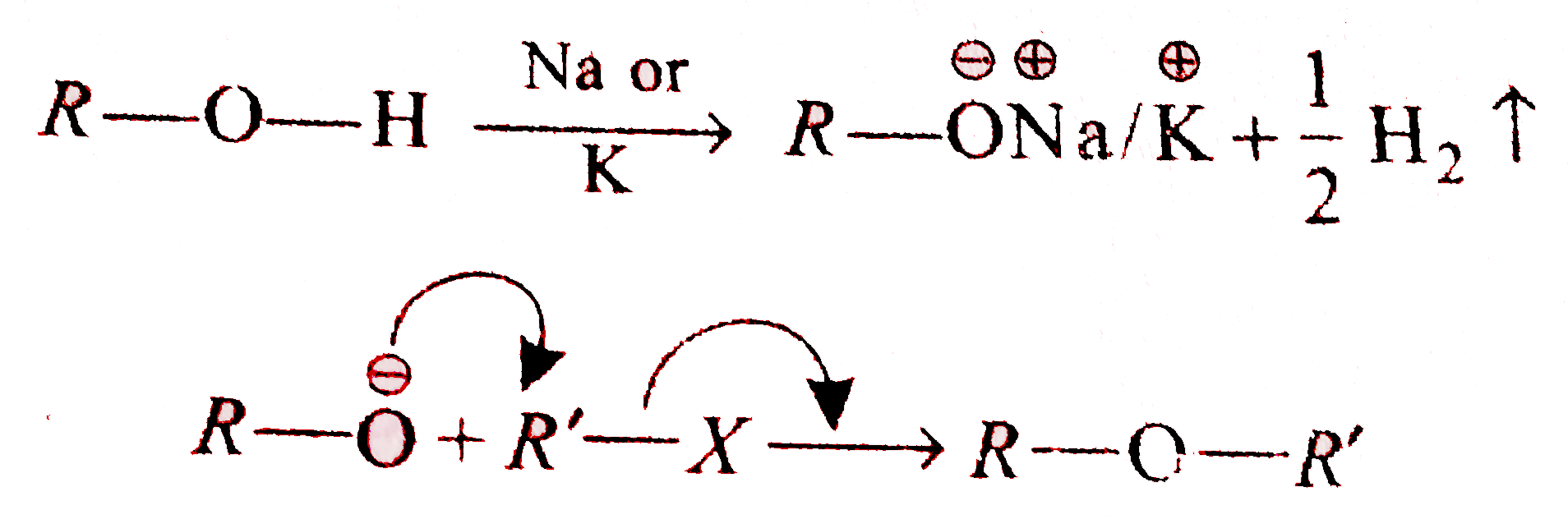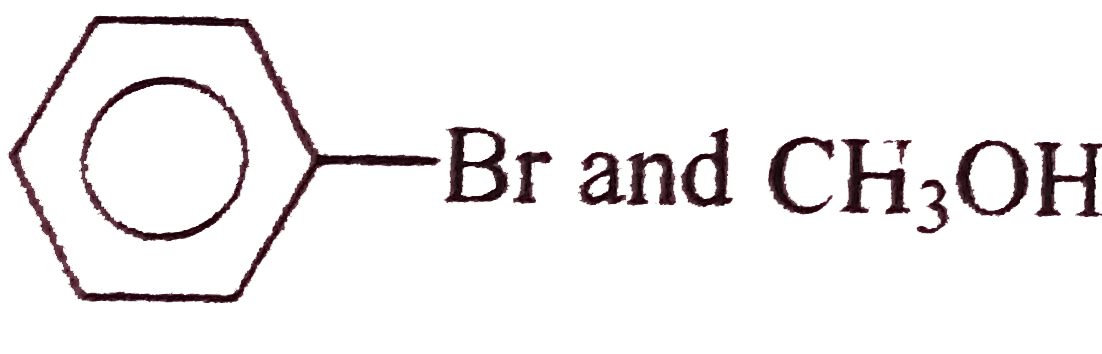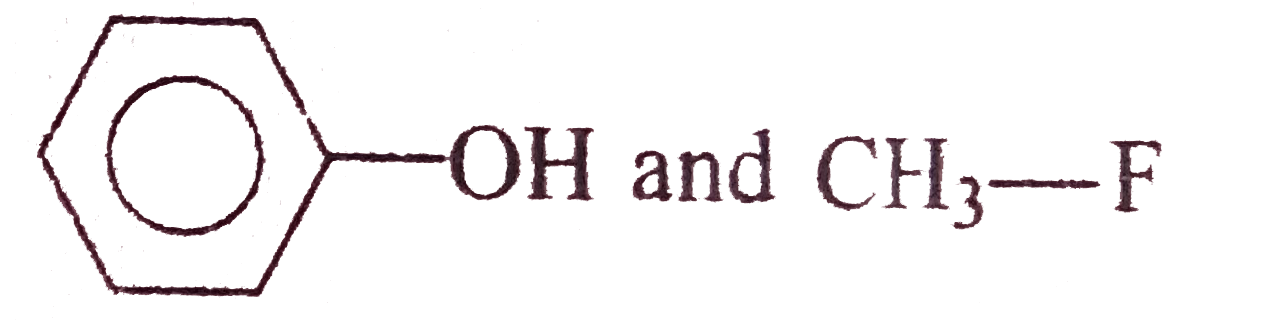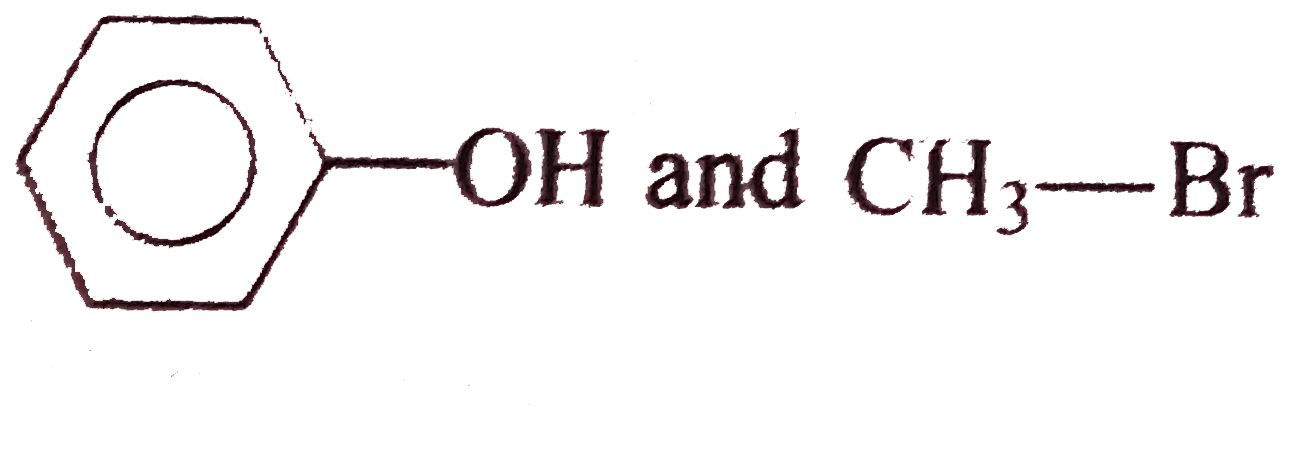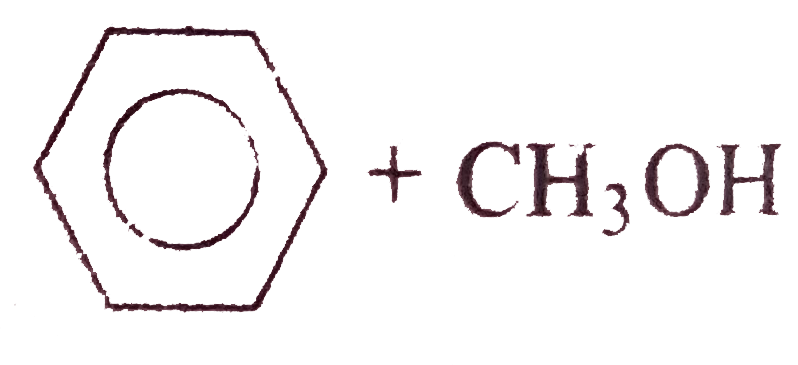A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALIDES
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.26 To Q.27)|2 VideosHALIDES
HIMANSHU PANDEY|Exercise Match The Column|13 VideosHALIDES
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.50)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosISOMERISM
HIMANSHU PANDEY|Exercise Subjective Type Problems|11 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HALIDES-Linked Comprehension Type (Q.1 To Q.25)
- R-X+Mgoverset(Ether,Delta)rarroverset(delta-)R-overset(delta+)Mg-X Gri...
Text Solution
|
- Since, Grignard reagents resemble carbanion, so they are strong nucleo...
Text Solution
|
- Since, Grignard reagents resemble carbanion, so they are strong nucleo...
Text Solution
|
- Since, Grignard reagents resemble carbanion, so they are strong nucleo...
Text Solution
|
- Consider the following sequence of reaction. Find structure of co...
Text Solution
|
- Consider the following sequence of reaction. Find structure of co...
Text Solution
|
- Consider the following sequence of reaction. Coverset(CF(3)CO(3)H...
Text Solution
|
- Williamson synthesis is an important method for the preparation of sym...
Text Solution
|
- Williamson synthesis is an important method for the preparation of sym...
Text Solution
|
- Williamson synthesis is an important method for the preparation of sym...
Text Solution
|
- Aliphatic nucleophilic substitution is mainly of two type S(N^(1))" an...
Text Solution
|
- Aliphatic nucleophilic substitution is mainly of two type S(N^(1))" an...
Text Solution
|
- Aliphatic nucleophilic substitution is mainly of two type S(N^(1))" an...
Text Solution
|
- Type of elimination reaction in which least substituted alkene is majo...
Text Solution
|
- Type of elimination reaction in which least substituted alkene is majo...
Text Solution
|
- Type of elimination reaction in which least substituted alkene is majo...
Text Solution
|
- There are number of organic compounds including esters, xanthate ester...
Text Solution
|
- There are number of organic compounds including esters, xanthate ester...
Text Solution
|
- There are number of organic compounds including esters, xanthate ester...
Text Solution
|
- Which one of the following is correct structure of 'B'?
Text Solution
|