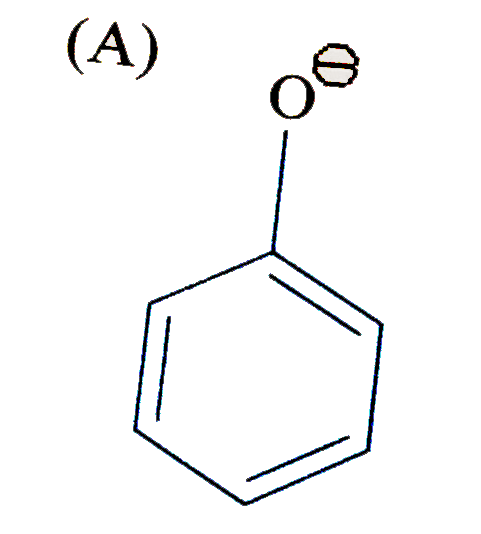
A
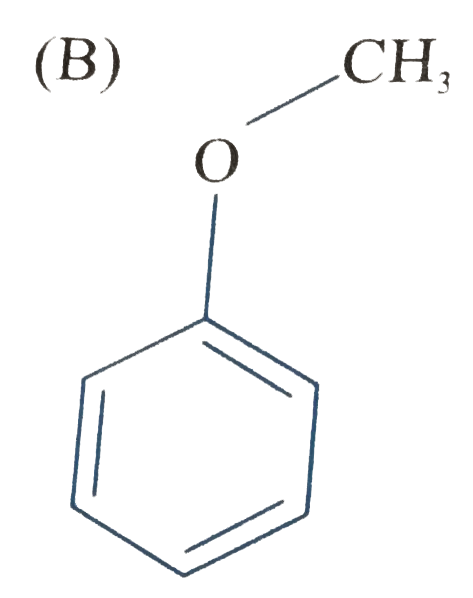
B
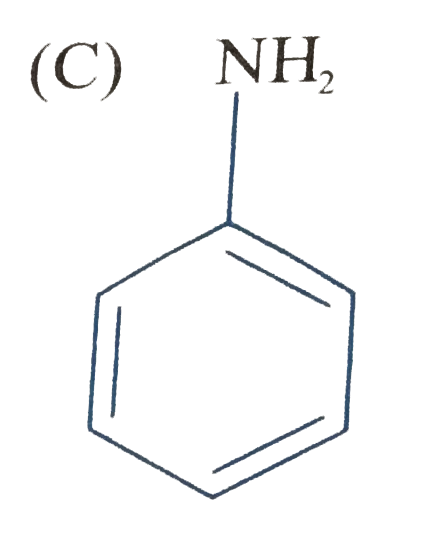
C
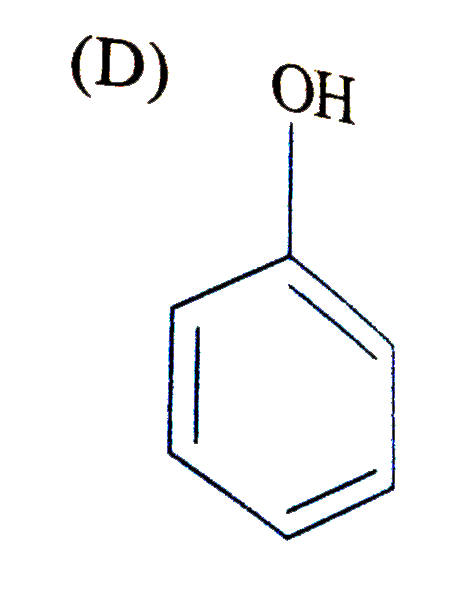
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-BENZENE-Level-6
- Major product above the reaction.
Text Solution
|
- In the following reaction, the structure of the major product (X) is:
Text Solution
|
- Which of the following is the most reactive towards eklectrophilic aro...
Text Solution
|
- The reaction of With HBr gives:
Text Solution
|
Text Solution
|
- Benzene on reaction with ICI in presence of anhydrous AlCl(3) gives?
Text Solution
|
- Complete the following reaction
Text Solution
|
- In which case, EAS will not be in meta position?
Text Solution
|
- Write the major product of the following reaction
Text Solution
|
- The compound (E) is:
Text Solution
|
- The product 'Y' is:
Text Solution
|
- Correct statement regarding the electrophilic substitution of C(6)H(6)
Text Solution
|
- The rate of the reaction depends on the concentration of
Text Solution
|
- Correct statements about this reaction
Text Solution
|
- In the above reaction
Text Solution
|
- Correct statement about this reaction is/are
Text Solution
|
- Generally rate of the reaction depends on the concentration of
Text Solution
|
- Major product. In this reaction.
Text Solution
|
- The correct statement(s) regarding the above reaction is/are
Text Solution
|
- Benzoic acid can be prepared by the oxidation of
Text Solution
|