Text Solution
Verified by Experts
The correct Answer is:
NARAYNA-BENZENE-Level-6
Text Solution
|
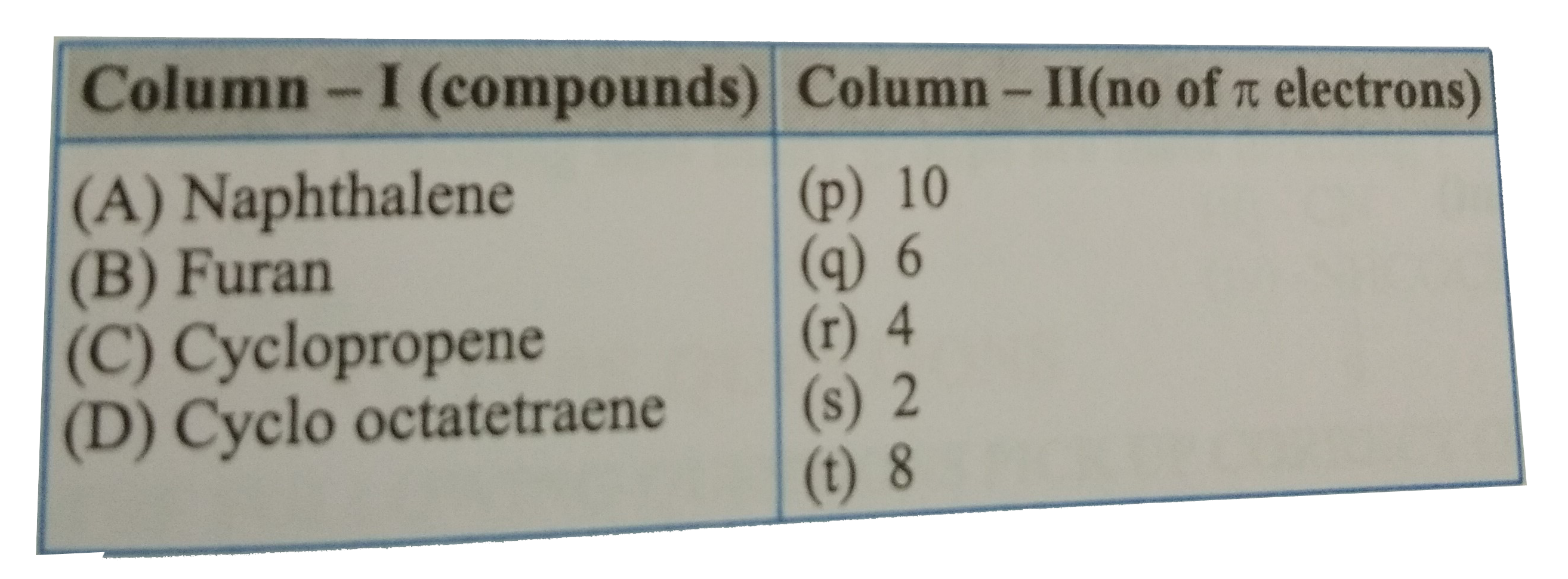
- Match the following .
Text Solution
|
- Match the following:
Text Solution
|
- how many species would be expected to exhibit aromatic character?
Text Solution
|
- Find out the number of reactions that are electrophilic aromatic subst...
Text Solution
|
- The possition number of 'Cl' in the major product of the reactio (loca...
Text Solution
|
- Z the position number of NO(2) Group X,Y,Z aer P,Q,R respectively. The...
Text Solution
|
- Products. The total number of pi bonds in the products is
Text Solution
|
- How many methods can be used for the preparation of iodobenzene? (i)...
Text Solution
|
- Complete the following reaction
Text Solution
|
- How many products are formed by the nitration of p-Xylene compound?
Text Solution
|
- How many methods can be used for the preparation of the isopropyl benz...
Text Solution
|
- Out of the following how many groups are meta directing? (i). -COOH ...
Text Solution
|
- Statement-I : Nitration of toluene is easier than benzene Because ...
Text Solution
|
- Statement-1: Tropylium cation is aromatic in nature Statement-2: Th...
Text Solution
|
- (A) Benene does not decolorise alkaline KMnO(4). ( R) Benzene is sta...
Text Solution
|
- What would e the major product in following reaction?
Text Solution
|
- When benzene is treated with DCl at low temperatures a compound C(6)H(...
Text Solution
|
- Accound for the following with ittle or no PhCH(2)(Br)CH(3))(2) forme...
Text Solution
|
- Complete the following reaction
Text Solution
|