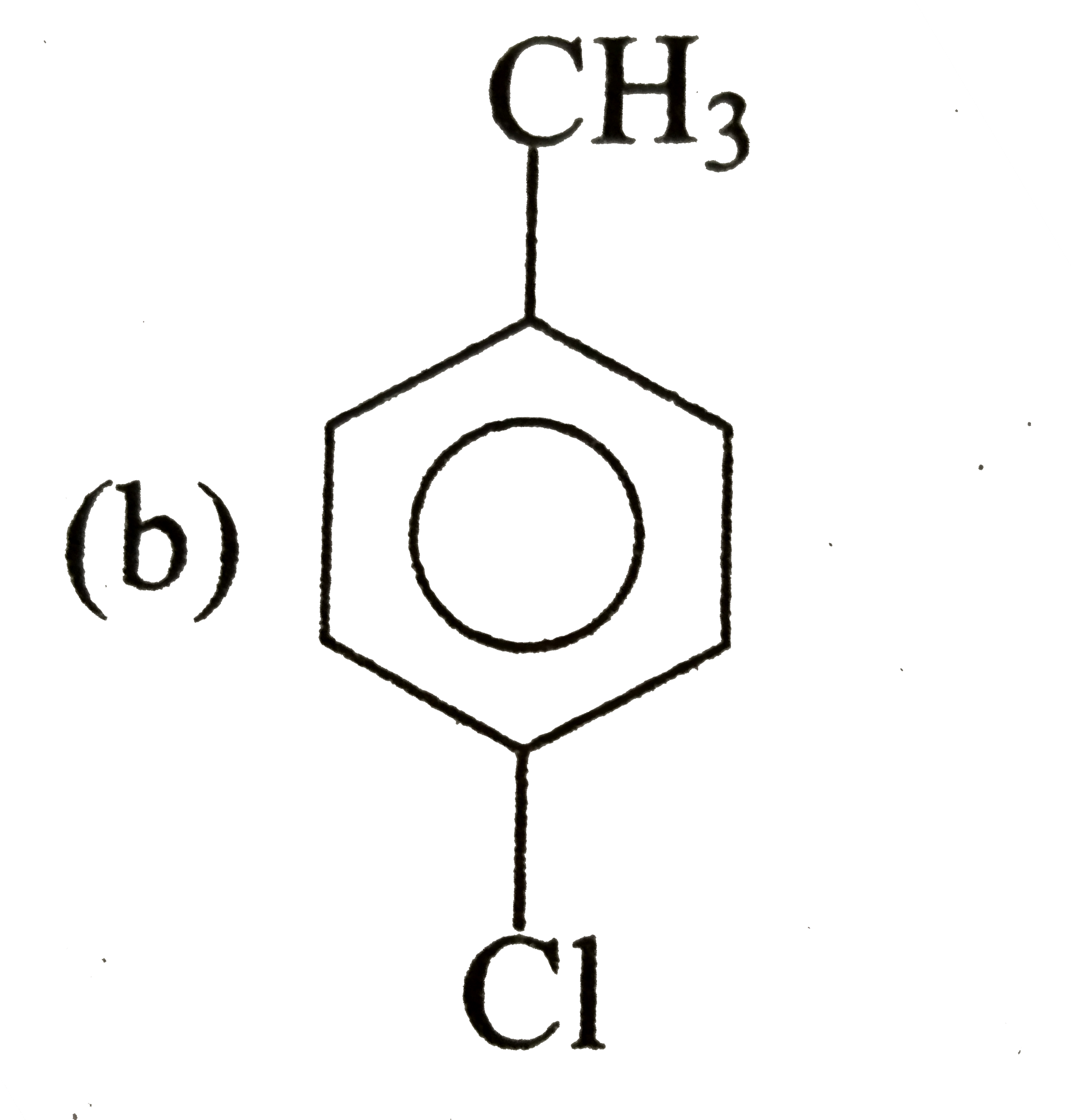
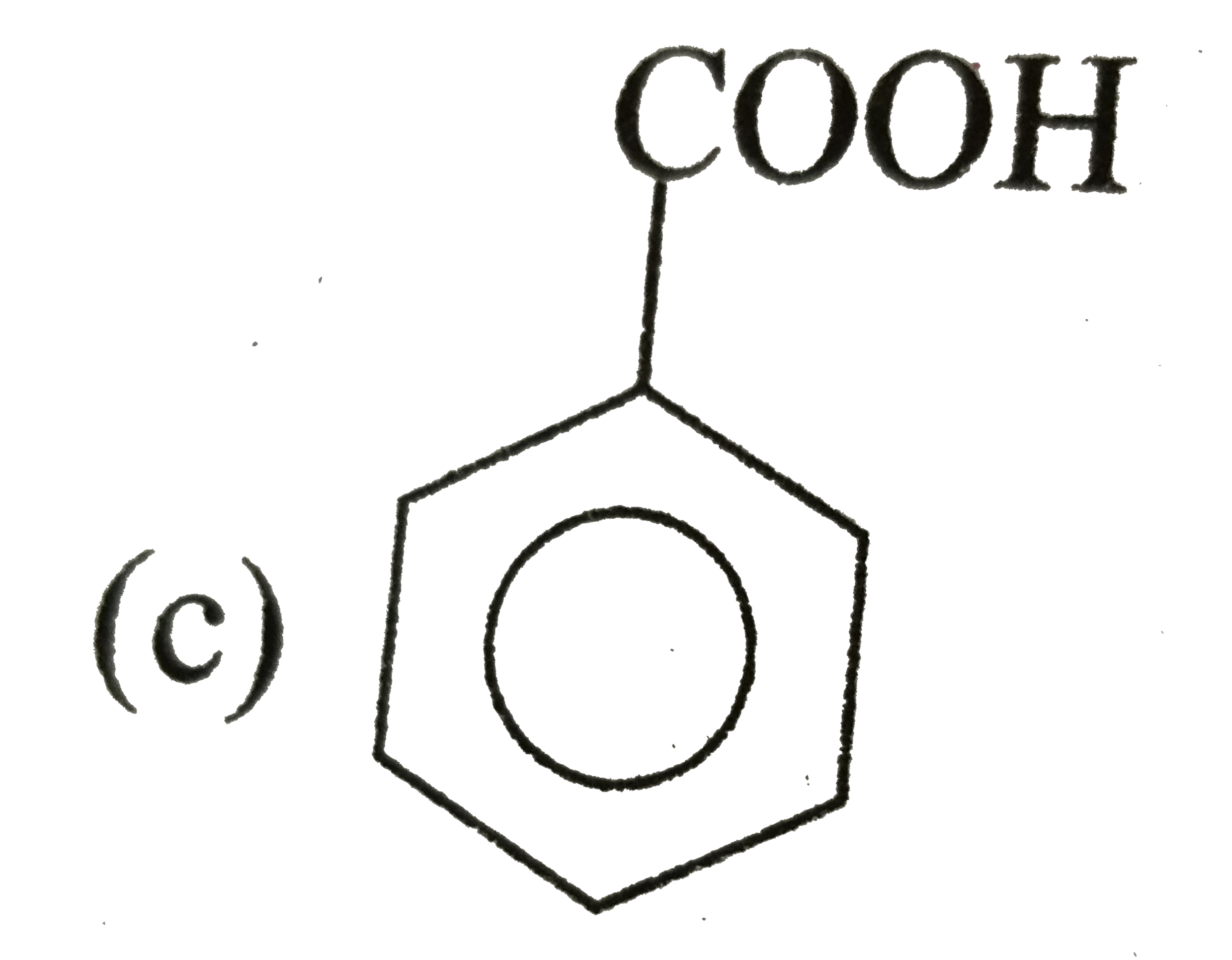
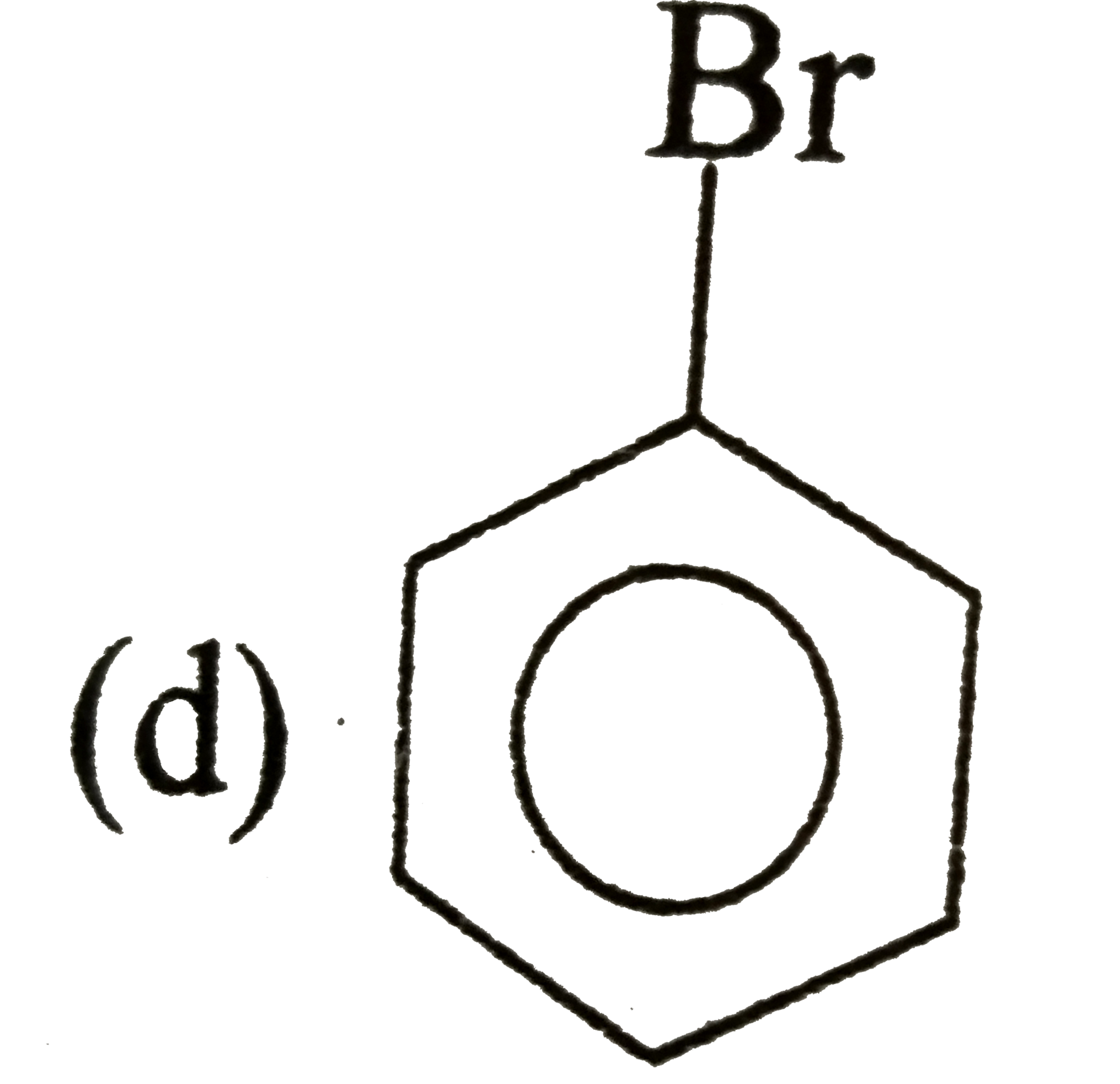
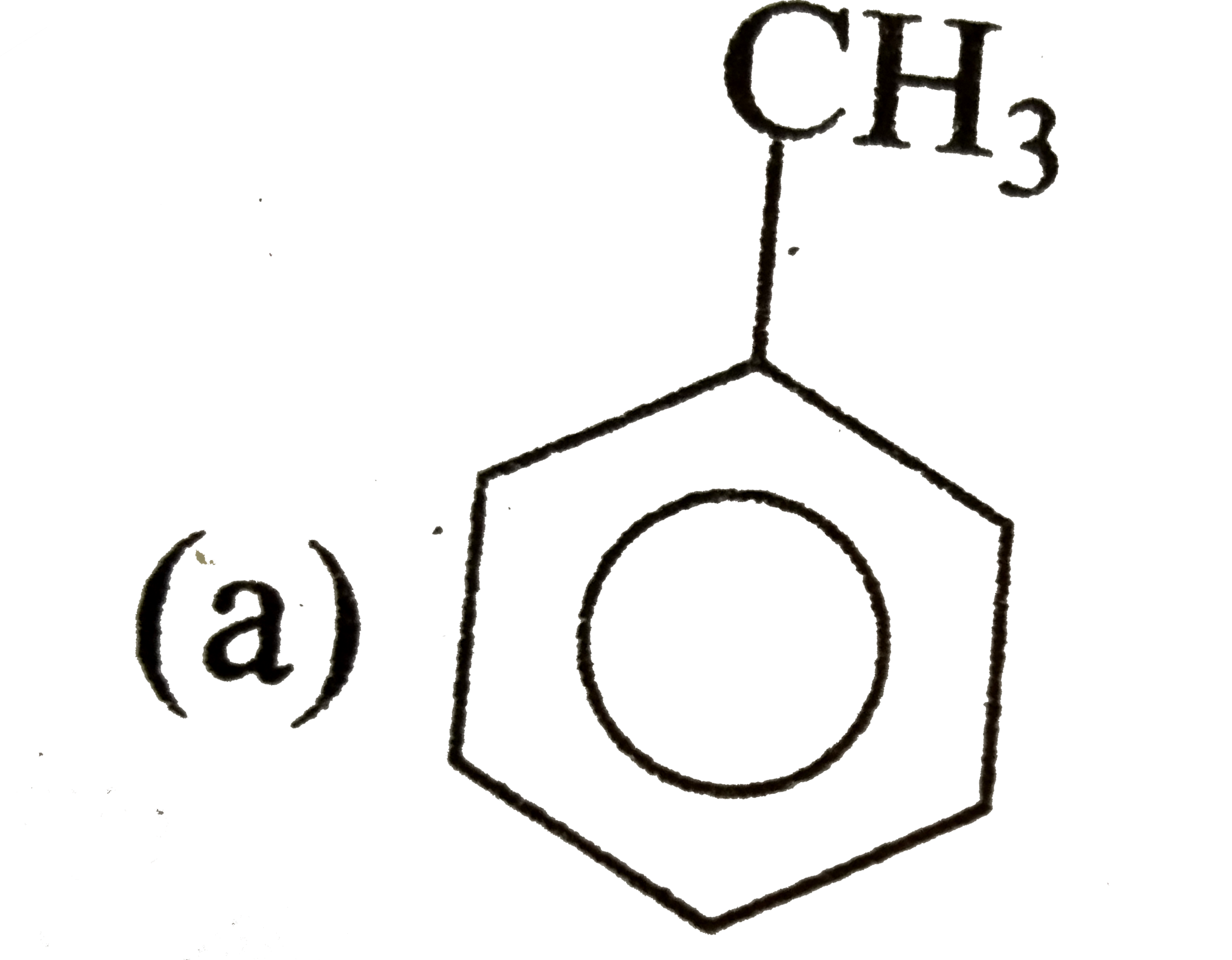A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.35)|10 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Linked Comprehension Type|25 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Level 3 (Q.151 To Q.160)|10 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-More Than One Correct (Q.1 To Q.25)
- Choose the compounds below that are non-aromatic :
Text Solution
|
- Choose the compounds below that are antiaromatic :
Text Solution
|
- Which of the following will undergo Friedel-Crafts alkylation reaction...
Text Solution
|
- Which pairs are not required for a nucleophilic aromatic substitution ...
Text Solution
|
- Halogens are deactivating yet ortho,para directing in electrophilic ...
Text Solution
|
- Which of the following are deactivating but orth, para
Text Solution
|
- Choose the correct statements :
Text Solution
|
- Identify the compounds that will undergo nucleophilic aromatic substit...
Text Solution
|
- Find out correct statement regarding nucleophilic aromatic substitutio...
Text Solution
|
- The reaction produces:
Text Solution
|
- Which of the following reactions
Text Solution
|
- The following conversion reaction can be carried out by using re...
Text Solution
|
- Iodobenzene can be obtained by :
Text Solution
|
- Complete the following reaction
Text Solution
|
- Which of the following are deacitvating group ?
Text Solution
|
- Which of the following are more reactive than diphenyl in electrophi...
Text Solution
|
- Diople moment of which compound is not zero ?
Text Solution
|
- Which of the following can be perpared by Remimer- Tiemann reaction di...
Text Solution
|
- Isopropyl benzene can be obtained by :
Text Solution
|
- Which of the following are pairs of antiaromatic species ?
Text Solution
|