Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER NORTH-2019
SUBHASH PUBLICATION|Exercise PART-D|27 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2019
SUBHASH PUBLICATION|Exercise PART-B|8 VideosANNUAL EXAMINATION QUESTION PAPER NORTH-2018
SUBHASH PUBLICATION|Exercise PART-E|8 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2017
SUBHASH PUBLICATION|Exercise PART-E|6 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER NORTH-2019-PART-C
- Define Electronegativity. How does it vary along a group?
Text Solution
|
- Which of the following will have most negative electron gain enthalpy?...
Text Solution
|
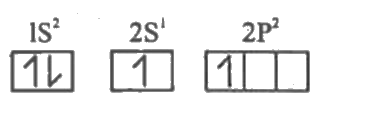
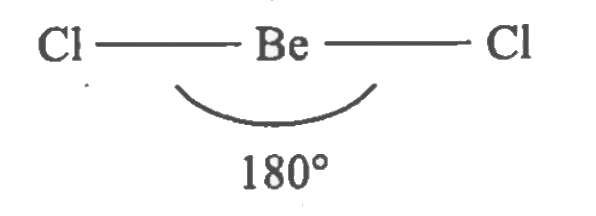
- Explain sp-hybridisation in BeCl2 molecule.
Text Solution
|
- Write any two differences between sigma-bond and Pi-bond.
Text Solution
|
- Name the type of Hydrogen bonding in ortho-nitrophenol.
Text Solution
|
- Write the Electronic configuration of Oxygen molecule. Predict its man...
Text Solution
|
- Balance the Redox -reaction by using Oxidation number method in acidic...
Text Solution
|
- How temporary Hardness of water is removed by Boiling?
Text Solution
|
- What is the composition of water gas?
Text Solution
|
- Complete the following reactions. CaCO(3)overset(1200K)(to)+
Text Solution
|
- Complete the following reactions. 2Ca(OH)(2)+2Cl(2)to ++
Text Solution
|
- Lithium shows diagonal relationship with Megnesium. Give reason.
Text Solution
|
- Write the chemical formula of Inorganic benzene.
Text Solution
|
- Write the dimeric structure of Aluminium chloride.
Text Solution
|
- Define catenation.
Text Solution
|
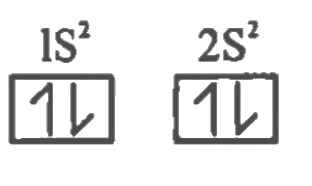 [Ground state]
[Ground state]