Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER SOUTH-2018
SUBHASH PUBLICATION|Exercise PART-B|9 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2018
SUBHASH PUBLICATION|Exercise PART-C|11 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2017
SUBHASH PUBLICATION|Exercise PART-E|6 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-E|7 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER SOUTH-2018-PART-E
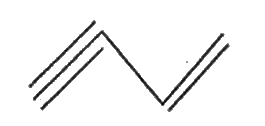
- Write the bond line structure of HC=C-CH=CH(2)
Text Solution
|
- Mention the IUPAC name of the following compound. underset(OH)unders...
Text Solution
|
- What is position isomerism? Give an example.
Text Solution
|
- Write the chemical equations when sodium fusion extract is prepared fr...
Text Solution
|
- Give two differences between inductive effect and electromeric effect.
Text Solution
|
- Write principles involved in estimatioin of halogen by Carius method.
Text Solution
|
- How is ethene prepared from bromoethane?
Text Solution
|
- Explain the mechanism of nitration of benzene .
Text Solution
|