Text Solution
Verified by Experts
Topper's Solved these Questions
ANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-D|19 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-E|7 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2019
SUBHASH PUBLICATION|Exercise PART-B|8 VideosANNUAL EXAMINATION QUESTION PAPER SOUTH-2018
SUBHASH PUBLICATION|Exercise PART-E|7 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
SUBHASH PUBLICATION|Exercise THREE MARKS QUESTIONS|28 Videos
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-ANNUAL EXAMINATION QUESTION PAPER SOUTH-2019-PART-C
- Define Electronegativity. How does it vary along a group?
Text Solution
|
- Explain sp^(3) hybridisation by taking methanae as example.
Text Solution
|
- Write the electronic configuration of Hydrogen molecule. Calculate its...
Text Solution
|
- Write any three postulates of VSEPR theory.
Text Solution
|
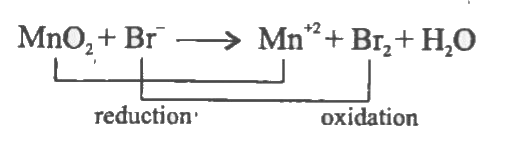
- Balance the following redox reaction by using oxidation number method....
Text Solution
|
- What are ionic hydrides? Give one example.
Text Solution
|
- What is temporary hardness of water?
Text Solution
|
- Explain the diagonal relationship between Lithium and Magnesium.
Text Solution
|
- Give the structure of diborane.
Text Solution
|