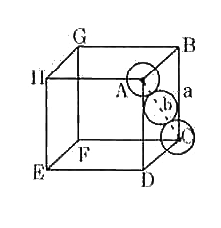
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THE SOLID STATE -PROBLEM SECTION
- Calculate packing efficiency in a CCP or HCP or FCC crystal lattice.
Text Solution
|
- Silver crystallizes in CCP lattice. The edge length of its unit cell i...
Text Solution
|
- An element having atomic mass 63.1 g/mol has face centered cubic unit ...
Text Solution
|
- An element having atomic mass 107.9 g mol^(-1) has FCC unit cell. The ...
Text Solution
|
- An element occurs in BCC structure with cell edte of 288 pm. Find the ...
Text Solution
|
- A compound formed by the element A and B crystallizes in the cubic str...
Text Solution
|
- An element crystallizes in fcc lattice. If the edge length of the unit...
Text Solution
|
- An element occurs in BCC structure with cell edge of 288 pm. It is 7.2...
Text Solution
|
- The density of chromium metal is 7.2 g cm^(-3). If the unit cell is cu...
Text Solution
|
- The density of an cubic unit cell is 10.5 g cm^(-3). If the edge lengt...
Text Solution
|
- Niobiumcrystallizs in body centered strucure. If density is 8.55 g cm^...
Text Solution
|
- Aluminium crystallizes in FCC structure. Atomic radius of the metalics...
Text Solution
|
- Sodium metal crystallizes in body centred cubic lattice with the cell ...
Text Solution
|
- An element with edge length 7Å crystallizes in SC. Calculate radius of...
Text Solution
|
- A compound A(x)B(y) crystallizes in a FCC lattice in which A occupies ...
Text Solution
|
- How many tetrahedral voids and octahedral voids are possible if the nu...
Text Solution
|
- A compound is formed by two elements x and y. Atoms of the element y (...
Text Solution
|