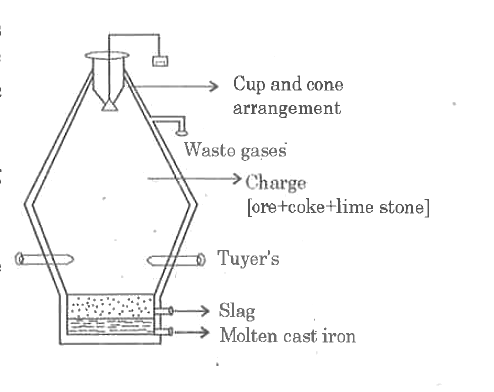
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS -Questions
- What is roasting ? Explain.
Text Solution
|
- What is calcination? Explain.
Text Solution
|
- What is the role of lime stone in the extraction of iron from the conc...
Text Solution
|
- Among carbon and carbon monoxide which one is a better reducing agent ...
Text Solution
|
- Among carbon and carbon monoxide which one is a better reducing agent ...
Text Solution
|
- What is the role of lime stone in the extraction of iron from the conc...
Text Solution
|
- Explain the process of obtaining 'blister copper' from copper matte'' ...
Text Solution
|
- Write the composition of copper matte.
Text Solution
|
- Name the flux used to remove iron impurity from molten copper matte.
Text Solution
|
- State the role of silica in the metallurgy of Copper.
Text Solution
|
- During the extraction of aluminium by Hall-Heroult process at which el...
Text Solution
|
- Draw labelled diagram of Hall-Heroult electrolytic cell for the extrac...
Text Solution
|
- In the extraction of aluminium by electrolysis. (i) Give the composi...
Text Solution
|
- During the extraction of aluminium by Hall-Heroult process at which el...
Text Solution
|
- In the reaction of gold with aquaregia, oxidation state of Nitrogen ch...
Text Solution
|
- Write the chemical reactions involved in the extraction gold using sod...
Text Solution
|
- Name any two ores of zinc. How is zinc extracted from zinc oxide?
Text Solution
|
- Name the reducing agent used in the extraction of zinc from zinc oxide...
Text Solution
|
- Explain electrolytic refining.
Text Solution
|
- Explain Zone refining.
Text Solution
|