Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THE 'P'-BLOCK ELEMENTS-EXERCISE
- Name the gas liberated when zinc reacts with dil HNO(3) .
Text Solution
|
- Name the metals which do not react with Conc. HNO(3) give reason.
Text Solution
|
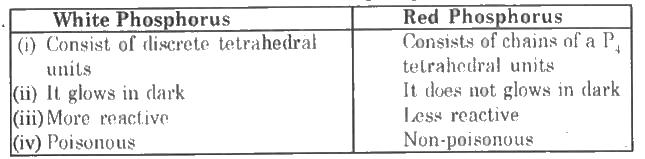
- Give two differences between white phosphorus and red phosphorus.
Text Solution
|
- How is phosphine prepared from calcium phosphide ?
Text Solution
|
- How phosphine is prepared in the laboratory ?
Text Solution
|
- Show that PH(3) is basic in nature.
Text Solution
|
- Give reason: PH(3) has lower melting point than NH(3)
Text Solution
|
- Give reason: Nitrogen is less reactive at room temperature.
Text Solution
|
- Which allotropic from of phosphorus has discrete tetrahedral P molecul...
Text Solution
|
- Bond angle in PH4+ is higher that in PH(3). Why?
Text Solution
|
- Name the acid obtained when PCl(5) undergoes hydrolysis, Give equatio...
Text Solution
|
- White phosphorous is heated with excess of dry chlorine to get X. X on...
Text Solution
|
- Are all the five bonds of PCl(5) equivalent ? Justify your answer.
Text Solution
|
- Write the structure for the following oxoacids of phosphorus. (i) Hy...
Text Solution
|
- How do you account of the reducing behaviour for H(3)PO(2) ?
Text Solution
|
- Mention any two reasons for the anomalous behaviour of oxygen.
Text Solution
|
- H(2)S is less acidic than H(2)Te. Give reason.
Text Solution
|
- Among the following which one is more acidic ? Give reason. H(2)O,H(...
Text Solution
|
- Why is H(2)O a liquid and H(2)S a gas?
Text Solution
|
- Give an example for amphoteric oxide.
Text Solution
|