Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THE 'P'-BLOCK ELEMENTS-EXERCISE
- White phosphorous is heated with excess of dry chlorine to get X. X on...
Text Solution
|
- Are all the five bonds of PCl(5) equivalent ? Justify your answer.
Text Solution
|
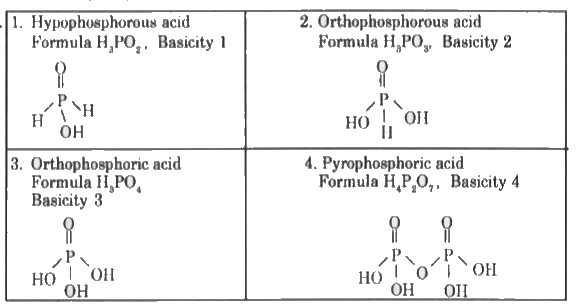
- Write the structure for the following oxoacids of phosphorus. (i) Hy...
Text Solution
|
- How do you account of the reducing behaviour for H(3)PO(2) ?
Text Solution
|
- Mention any two reasons for the anomalous behaviour of oxygen.
Text Solution
|
- H(2)S is less acidic than H(2)Te. Give reason.
Text Solution
|
- Among the following which one is more acidic ? Give reason. H(2)O,H(...
Text Solution
|
- Why is H(2)O a liquid and H(2)S a gas?
Text Solution
|
- Give an example for amphoteric oxide.
Text Solution
|
- Describe the preparation of Ozonised oxygen with an equation. Name the...
Text Solution
|
- Give two examples to show that ozone is in oxidising agent.
Text Solution
|
- Write the equation for the action of ozone with lead sulphide.
Text Solution
|
- Mention the allotropic form of sulphur which is more stable above 369 ...
Text Solution
|
- Complete the following equation NO + O(3)rarr
Text Solution
|
- Give the conversation of (i) SO(2) " to " SO(2)Cl(2)
Text Solution
|
- Give the conversation of (i) SO(2) " to " SO(3)
Text Solution
|
- Given two examples to show that moist SO(2) is a reducing agent.
Text Solution
|
- Complete the following equation. 2Fe^(3+)+SO(2)+2H(2)Orarr
Text Solution
|
- Give the structure for (a) Sulphurous acid (b) Sulphuric acid (...
Text Solution
|
- How is Cone. H(2)SO(4) manufactured by contact process ?
Text Solution
|