Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THE 'P'-BLOCK ELEMENTS-EXERCISE
- Given two examples to show that moist SO(2) is a reducing agent.
Text Solution
|
- Complete the following equation. 2Fe^(3+)+SO(2)+2H(2)Orarr
Text Solution
|
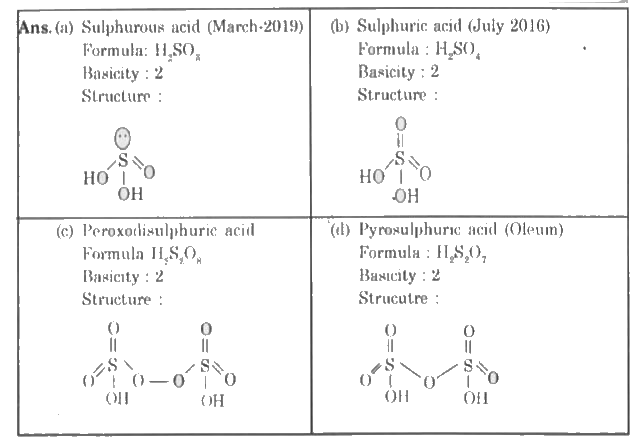
- Give the structure for (a) Sulphurous acid (b) Sulphuric acid (...
Text Solution
|
- How is Cone. H(2)SO(4) manufactured by contact process ?
Text Solution
|
- Complete the following equation. H(2)SO(4)+So(3)rarr?
Text Solution
|
- Show that Conc. H(2)SO(4) is a dehydrating agent.
Text Solution
|
- Complete the following equation. C(12)H(22)O(11)overset(H(2)SO(4))ra...
Text Solution
|
- Complete the following equation and balance . * Cu+H(2)SO(4)rarr *...
Text Solution
|
- Given two anomalous properties of fluorine
Text Solution
|
- Fluorine exhibits only - 1 oxidation state , whereas other halogens ex...
Text Solution
|
- Complete the following equations . 2F(2)+2H(2)Orarr
Text Solution
|
- Complete the following equations. 2PbO(2)(s)overset(heat) rarr……….+…...
Text Solution
|
- Give three methods of preparation of chlorine gas.
Text Solution
|
- Name the gas liberated when concentrated HCI is heated with MnO(2) Giv...
Text Solution
|
- How is chlorine prepared in the laboratory using KMNo(4) ?
Text Solution
|
- How chlorine gas is manufactured by Deacon's process ?
Text Solution
|
- Give three examples to show that chlorine has affinity towards hydroge...
Text Solution
|
- How excess of ammonia reacts with chorine .
Text Solution
|
- How excess of chlorine reacts with ammonia.
Text Solution
|
- How conc and dilute sodium hydroxide ( alkali ) reacts with chlorine.
Text Solution
|