Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
SUBHASH PUBLICATION-THE 'P'-BLOCK ELEMENTS-EXERCISE
- Complete the following reactions. 2FeSO(4)+H(2)SO(4)+Cl(2)rarr
Text Solution
|
- Complete the following reactions. Cl(2)+3F(2)rarr
Text Solution
|
- Complete the equation SO(2)+Cl(2)+2H(2)Orarr?
Text Solution
|
- Which halogen has highest electron affinity or electron gain enthalpy ...
Text Solution
|
- Show that chlorine is a bleaching agent.
Text Solution
|
- What is an aqua regia ? Give its one use.
Text Solution
|
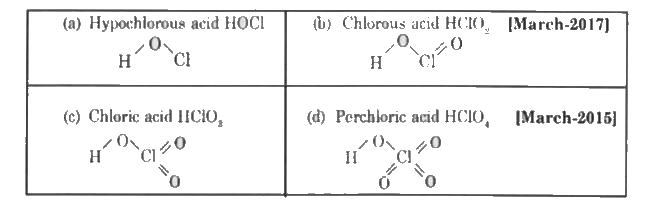
- Give the structure of ( a ) Hypochlorous acid ( b ) chlorous acid ( ...
Text Solution
|
- Which is the strongest acid among the hydrogen halides? Give one reaso...
Text Solution
|
- Give reason ''BrF(5) Is more reactive than Br(2)''
Text Solution
|
- What is the shape of ClF(3),BrF(5),IF(7) molecules.
Text Solution
|
- Interhalogen compounds are more reactive than halogens. Give reason.
Text Solution
|
- Why are the elements of Group - 18 known as noble gases?
Text Solution
|
- What is the commercial sources of helium?
Text Solution
|
- Complete the following equation. .(88)^(226)Rararr.(88)^(226)Rn+.........
Text Solution
|
- Noble gases have vary low boiling point. Why?
Text Solution
|
- Give reason for chemical inertness of noble gases.
Text Solution
|
- Name the noble gas which does not have general noble gas configuration...
Text Solution
|
- Name the noble gas obtained as a decay product of..^(226)R
Text Solution
|
- Complete the following equation.
Text Solution
|
- Write the general electronic configuration of noble gases.
Text Solution
|