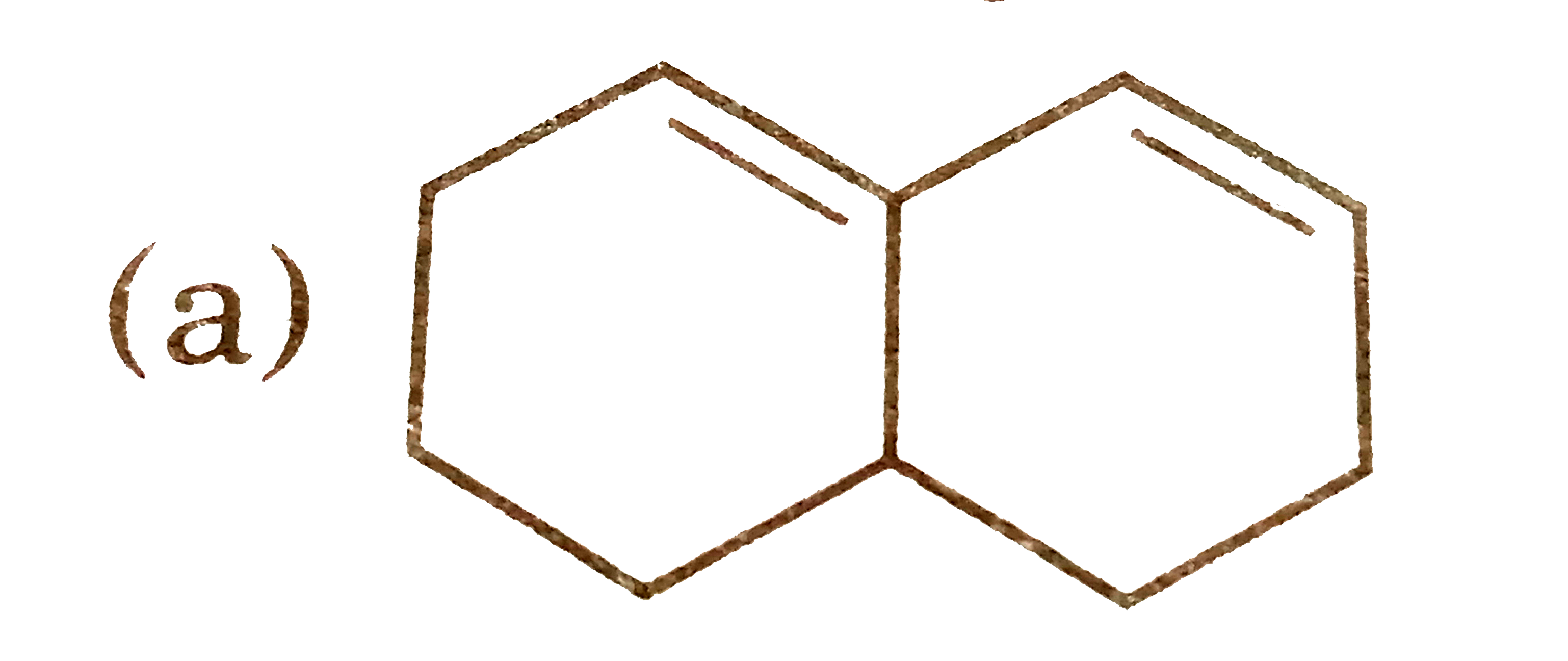
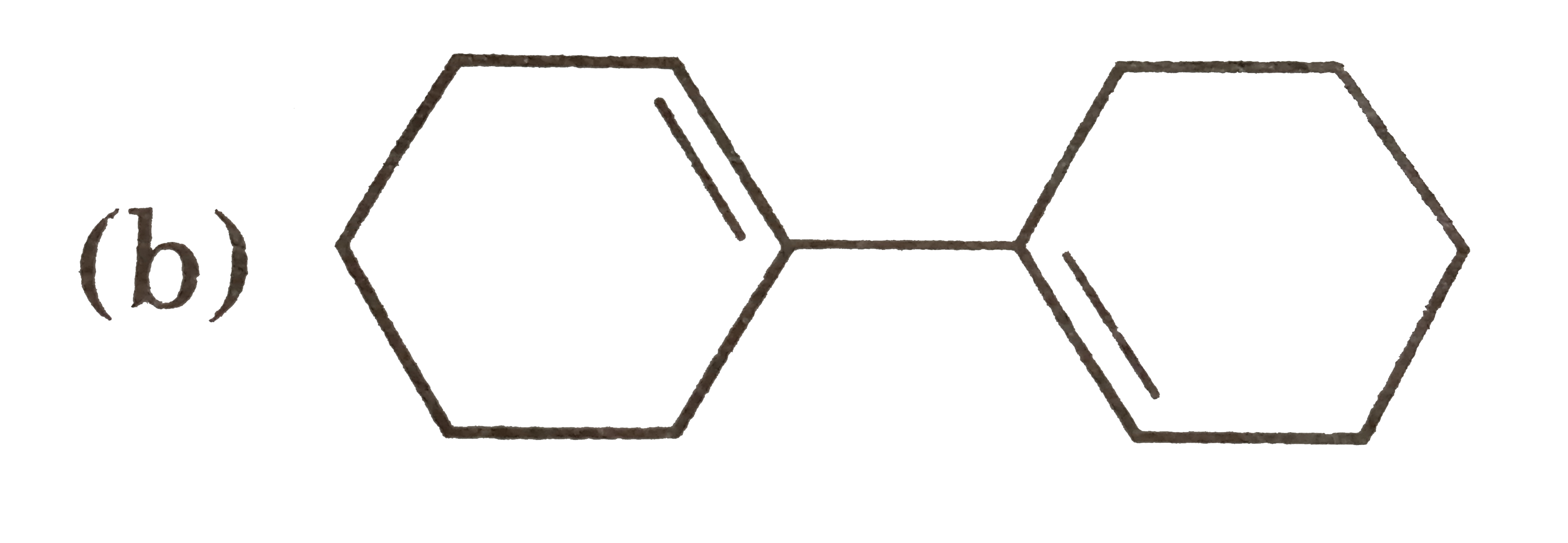
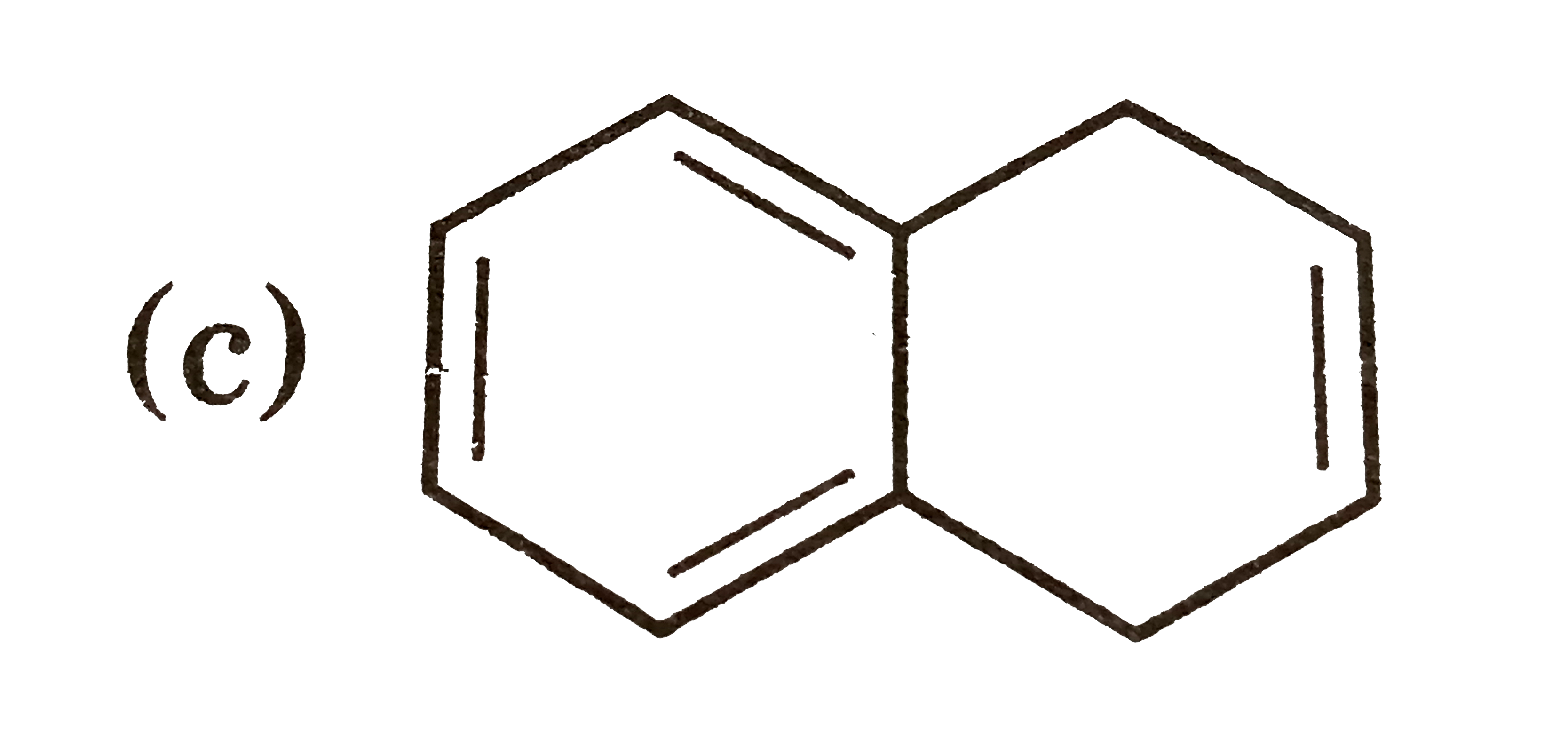
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Reasoning Type|15 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Multiple Objective Type|56 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Subjective Type|23 VideosHYDROGEN AND ITS COMPOUNDS
GRB PUBLICATION|Exercise Subjective type|8 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-HYDROCARBON (ALIPHATIC)-Previous years jee questions
- Which of following hydrocarbon can react with maleic anhydride?
Text Solution
|
- Which of the following will decolourise alkaline KMnO(4)?
Text Solution
|
- The compound in which the distance between the two adjacent carbon ato...
Text Solution
|
- Which of the following compounds does not dissolve in conc. H(2)SO(4) ...
Text Solution
|
- Anti-Markownikoff's addition of HBr is not observed in-
Text Solution
|
- The reaction conditions leading to the best yield of C(2)H(5)Cl are
Text Solution
|
- n-Propyl bromide on treatment with ethanolic potassium hydroxide produ...
Text Solution
|
- The major product of reaction between n-butane and bromine at 130^(@)C...
Text Solution
|
- During debromination of meso- dibromobutane, the major compound forme...
Text Solution
|
- The intermediate formed during the addition of HCl to propene in the p...
Text Solution
|
- The product (s) via - oxymercuration (HgSO(4)+H(2)SO(4)) of 1- butyne...
Text Solution
|
- Propyne and propene can be distinguished by :
Text Solution
|
- Which of the following alkenes will react fastest with H(2) under cata...
Text Solution
|
- In the presence of peroxide, hydrogen chloride and hydrogen iodide do ...
Text Solution
|
- Hydrogenation of the above compound in the presence of poisoned Pd cat...
Text Solution
|
- The addition of propene with HOCl proceeds via the addition of:
Text Solution
|
- The nodal plane in the pi-bond of ethene is located in:
Text Solution
|
- Consider the following reaction: CH(3)underset(D)underset(|)CH-under...
Text Solution
|
- Identify a reagent from the following list which can easily distinguis...
Text Solution
|
- Ph-Cequiv C-CH(3)overset(Hg^(2+)//H^(+))toA, A is
Text Solution
|
- 2-Hexyne gives trans -2- hexene on treatment with :
Text Solution
|