A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
HYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Comprehension Type-1|3 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Comprehension Type-2|3 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Reasoning Type|15 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Subjective Type|23 VideosHYDROGEN AND ITS COMPOUNDS
GRB PUBLICATION|Exercise Subjective type|8 Videos
GRB PUBLICATION-HYDROCARBON (ALIPHATIC)-Multiple Objective Type
- Methane will be produced in which of the following reaction:
Text Solution
|
- Complete the following reaction
Text Solution
|
- Select correct statement:
Text Solution
|
- Aoverset(O(3))underset(Me(2)S)(to)H-overset(O)overset(||)C-CH(2)CH(2)-...
Text Solution
|
- Which of the following reaction can proceed via formation of a 6 membe...
Text Solution
|
- HgSO(4)//H(2)SO(4) " and " B(2)H(6)//NaOH .H(2)O(2) will give same ma...
Text Solution
|
- Which of the following represent correct product formation?
Text Solution
|
- Incorrect about the product:
Text Solution
|
- Suitable reagent (s) to carry out following conversion is/are
Text Solution
|
- Which of the following reactants will give same product with HBr in p...
Text Solution
|
- CH(3)-CH=CH(2)+Cl(2)+" Methanolic NaBr " to "product(s)" Which produ...
Text Solution
|
- When an aqueous solution containing MeCOOK and Me(3)C-COOK is electrol...
Text Solution
|
- Choose all alkane that give only one monochloro derivative upon reacti...
Text Solution
|
- Which of the following hydrocarbons can be made by using single reacta...
Text Solution
|
- Which of the following is//are possible product(s) of given reaction?
Text Solution
|
- overset(14)(C)H(2)=CH-CH(3)overset(NBS)underset(C Cl(4),Delta)(to)over...
Text Solution
|
- Which of the following reaction (s) leads to new C-C bond formation in...
Text Solution
|
- Which of the following statement is True
Text Solution
|
- Monochlorination of (S)-2-chlorobutane can give:
Text Solution
|
- Select option (s) in which incorrect major product is shown:
Text Solution
|
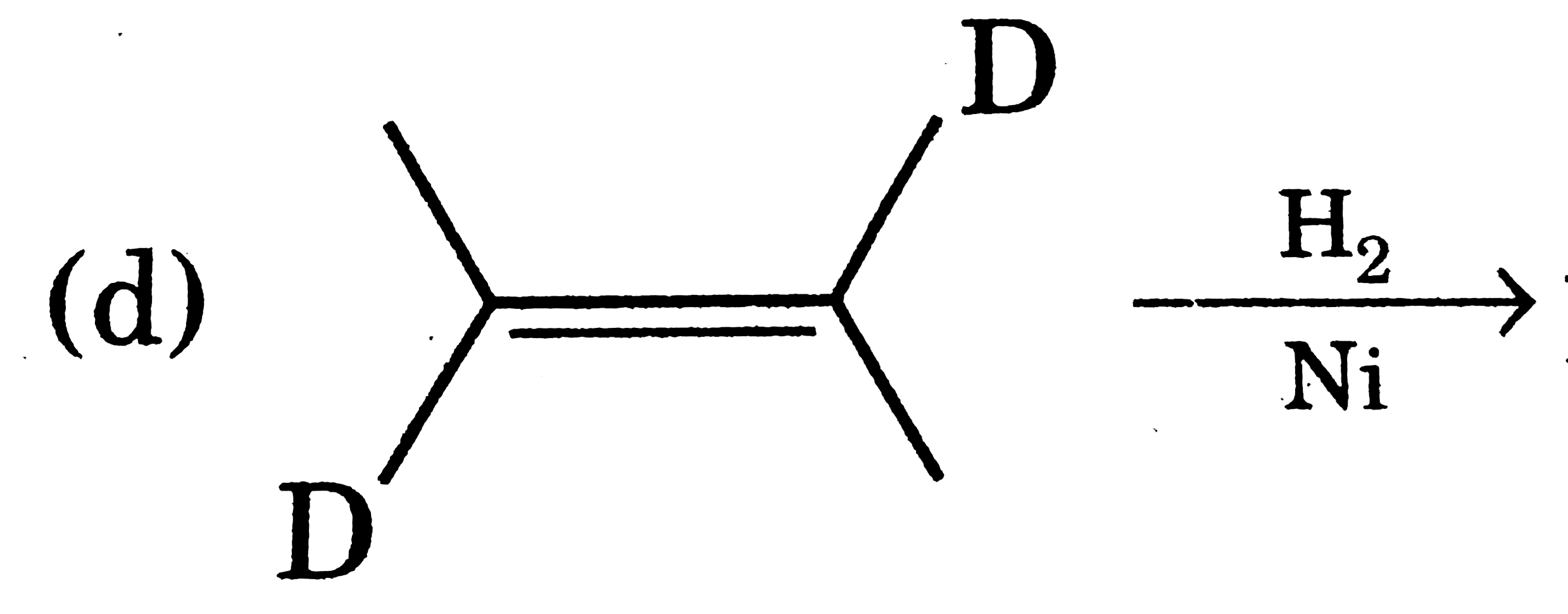 Product optained show no optical rotation
Product optained show no optical rotation