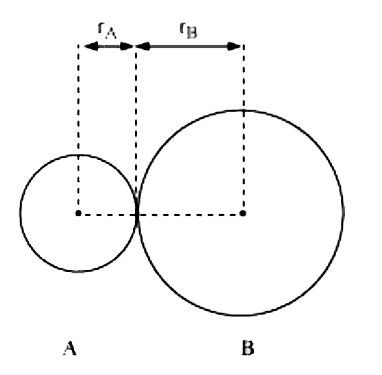
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-CHEMICAL BONDING AND MOLECULAR STRUCTURE-EXERCISE
- Although geometries of NH(3) and H(2)O molecules are distorted tetrahe...
Text Solution
|
- How do you express the bond strength in terms of bond order?
Text Solution
|
- Define the bond length.
Text Solution
|
- Explain the important aspects of resonance with reference to the CO(3)...
Text Solution
|
- H(3)PO(3) can be represented by structure (a) and (b) shown below. Can...
Text Solution
|
- Write the resonance structures for SO(3),NO(2), and NO(3)^(ө).
Text Solution
|
- Use Lewis symbols to show electron transfer between the following atom...
Text Solution
|
- Although both CO(2) and H(2)O are triatomic molecules, the shape of H(...
Text Solution
|
- Write the significance/applications of dipole moment
Text Solution
|
- Define electronegativity. How does it differ from electron gain enthal...
Text Solution
|
- Explain with the help of suitable example polar covalent bond.
Text Solution
|
- Arrange the bonds in order of increasing ionic character in the molecu...
Text Solution
|
- The skeletal structure of CH(3)COOH as shown below is correct, but som...
Text Solution
|
- Apart from tetrahedral geometry, another possible geometry for CH(4) i...
Text Solution
|
- Explain why BeH(2) molecule has a zero dipole moment although the Be-H...
Text Solution
|
- Out of NH(3) and NF(3) which has a higher Dipole moment?
Text Solution
|
- What is meant by hybridisation of atomic orbitals? Describe the shape ...
Text Solution
|
- Describe the change in hybridization (if any) of the Al atom in the fo...
Text Solution
|
- Is there any change in hybridisation of the B and N atom as a result o...
Text Solution
|
- Draw diagrams showing the formation of a double bond and a triple bond...
Text Solution
|