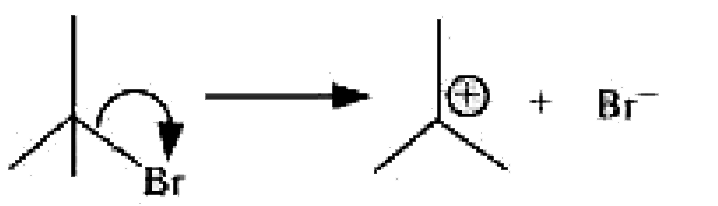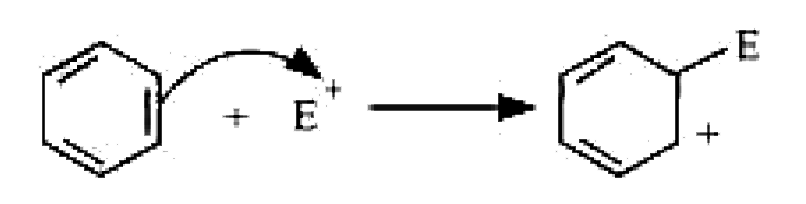Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUES-EXERCISE
- Classify the following reactions in one of the reaction type studied i...
Text Solution
|
- What is the relationship between the members of the following pairs of...
Text Solution
|
- For the following bond cleavages, use curved-arrow to show the electro...
Text Solution
|
- Explain the terms Inductive and Electromeric effects. Which electron d...
Text Solution
|
- Give a brief description of the principles of the following techniques...
Text Solution
|
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|
- Discuss the chemistry of Lassaigne’s test.
Text Solution
|
- Differentiate between the principle of estimation of nitrogen in an or...
Text Solution
|
- Discuss the principle of estimation of halogens, sulphur and phosphoru...
Text Solution
|
- Explain the principle of paper chromatography.
Text Solution
|
- Why is nitric acid added to sodium extract before adding silver nitrat...
Text Solution
|
- Explain the reason for the fusion of an organic compound with metallic...
Text Solution
|
- Name a suitable technique of the components from a mixture of calcium ...
Text Solution
|
- Explain why an organic liquid vaporises at a temperature below its boi...
Text Solution
|
- Will C Cl4 give white precipitate of AgCl on heating with nitrate? Giv...
Text Solution
|
- Why is solution of potassium hydroxide used to absorb carbon dioxide e...
Text Solution
|
- Why is it necessary to use acetic acid and not suplhuric acid for the ...
Text Solution
|
- An organic compound contains 69% carbon and 4.8% hydrogen, the remaind...
Text Solution
|
- 0.50 gm of an organic compound was treated according to Kjeldahl's meg...
Text Solution
|
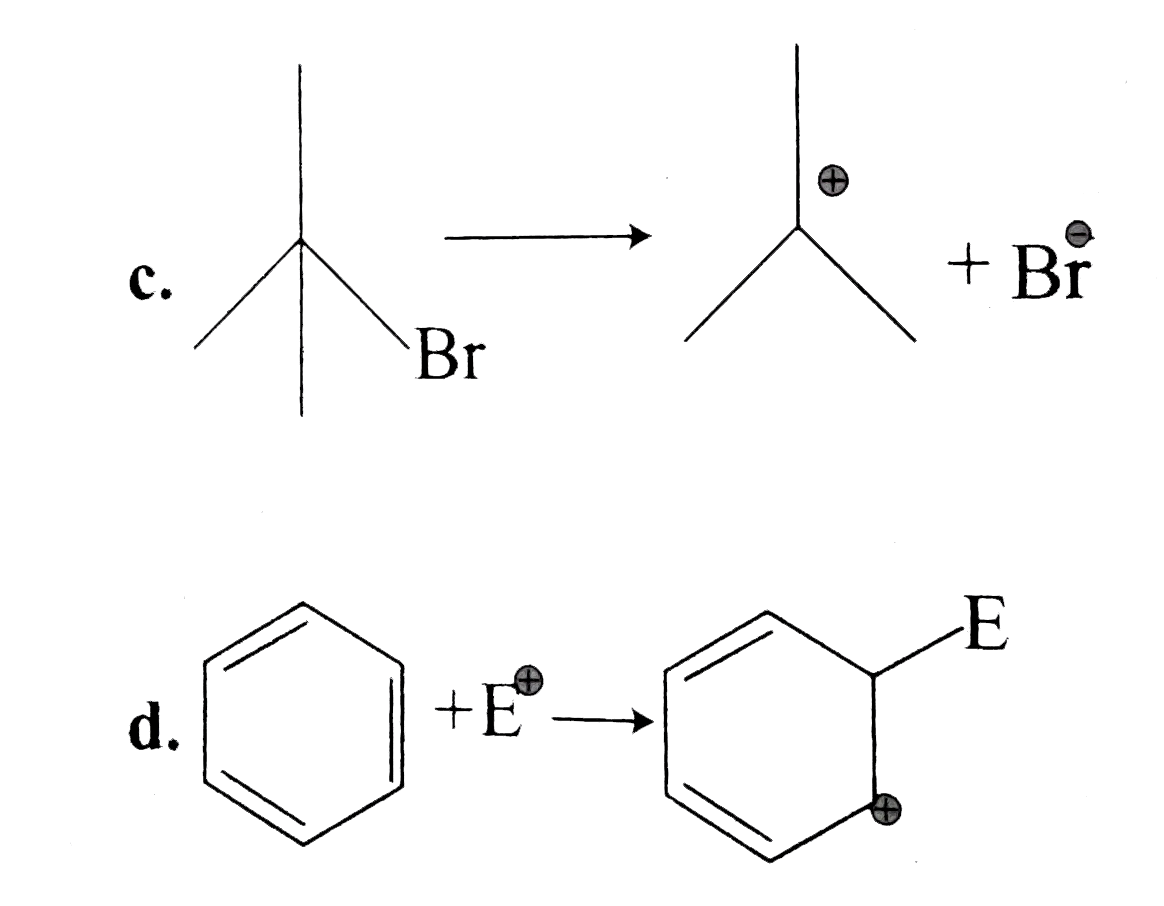 .
.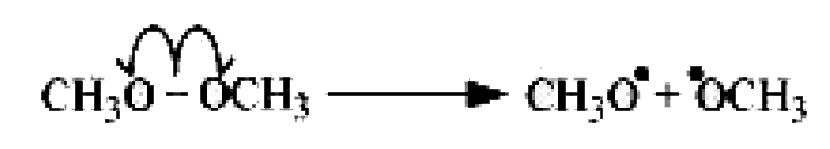
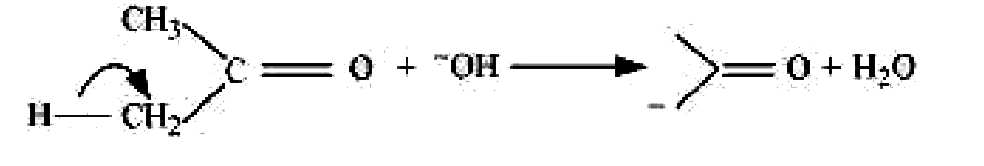 It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate formed is carbanion.
It is an example of heterolytic cleavage as the bond breaks in such a manner that the shared pair of electrons remains with the carbon of propanone. The reaction intermediate formed is carbanion.