Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ORGANIC CHEMISTRY- SOME BASIC PRINCIPLES AND TECHNIQUES-EXERCISE
- Describe the method, which can be used to separate two compounds with ...
Text Solution
|
- What is the difference between distillation, distillation under reduce...
Text Solution
|
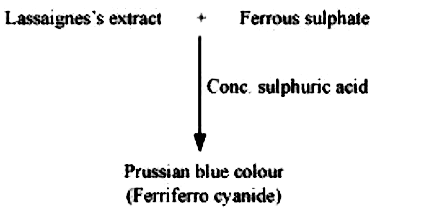
- Discuss the chemistry of Lassaigne’s test.
Text Solution
|
- Differentiate between the principle of estimation of nitrogen in an or...
Text Solution
|
- Discuss the principle of estimation of halogens, sulphur and phosphoru...
Text Solution
|
- Explain the principle of paper chromatography.
Text Solution
|
- Why is nitric acid added to sodium extract before adding silver nitrat...
Text Solution
|
- Explain the reason for the fusion of an organic compound with metallic...
Text Solution
|
- Name a suitable technique of the components from a mixture of calcium ...
Text Solution
|
- Explain why an organic liquid vaporises at a temperature below its boi...
Text Solution
|
- Will C Cl4 give white precipitate of AgCl on heating with nitrate? Giv...
Text Solution
|
- Why is solution of potassium hydroxide used to absorb carbon dioxide e...
Text Solution
|
- Why is it necessary to use acetic acid and not suplhuric acid for the ...
Text Solution
|
- An organic compound contains 69% carbon and 4.8% hydrogen, the remaind...
Text Solution
|
- 0.50 gm of an organic compound was treated according to Kjeldahl's meg...
Text Solution
|
- 0.3080 gm of and organic chloro compound gave 0.5740 gm of siver chlor...
Text Solution
|
- In the estimation of sulphur by carius method, 0.468 gm of an organic ...
Text Solution
|
- In the organic compound CH(2) = CH – CH(2) – CH(2) – C equivCH, the pa...
Text Solution
|
- In the Lassaigne’s test for nitrogen in an organic compound, the Pruss...
Text Solution
|
- Which of the following carbocation is most stable ? (a) (CH(3))(3)C....
Text Solution
|