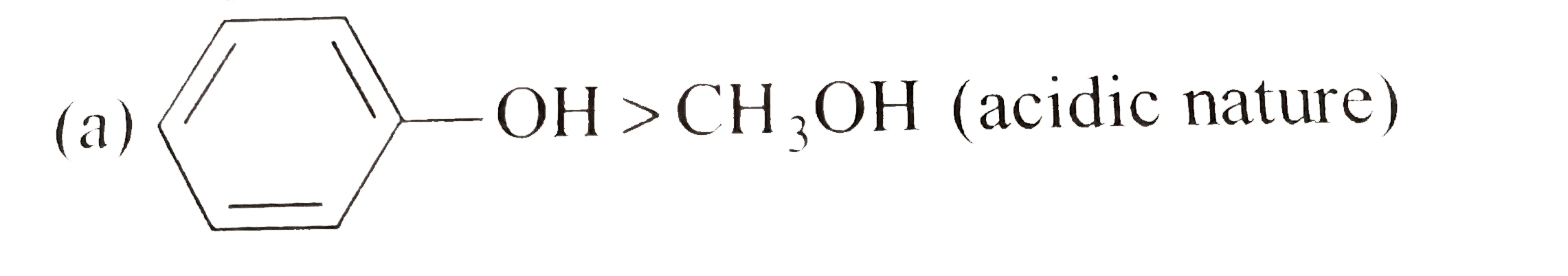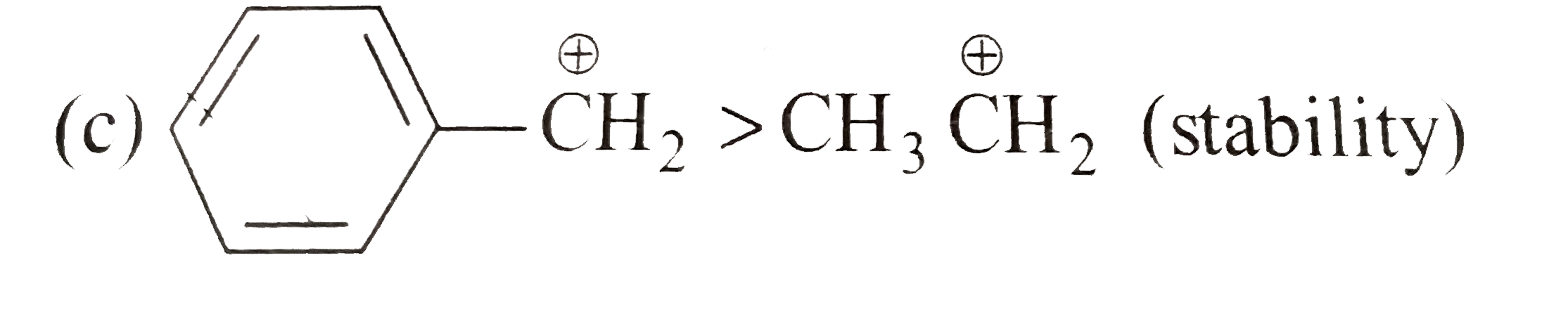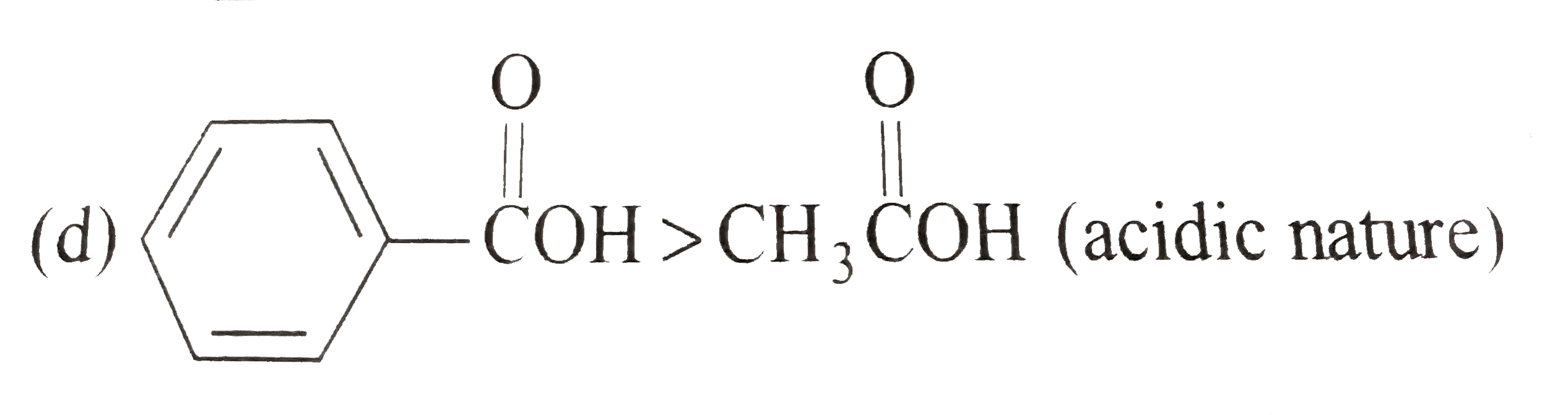A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.1 To Q.25)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.26 To Q.50)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Subjective Type Problems|19 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 1 (Q.26 To Q.49)
- In which of the following molecules pi-electron density in ring is min...
Text Solution
|
- In which of the following compounds the hydroxuylic protion is most ac...
Text Solution
|
- The correct order of acidity of the following is :
Text Solution
|
- In the following compounds, the order of basicity is :
Text Solution
|
- Consider The correct order of their acidity is :
Text Solution
|
- The correct order of decreasing basicity of the above compounds is :
Text Solution
|
- Select the correct order of basicity .
Text Solution
|
- The acidity of the protons H^(+) in the following is : (I) CH(3)CH(2...
Text Solution
|
- Which comparison is not correct as indicated ?
Text Solution
|
- How many pi electrons are there in the following species ?
Text Solution
|
- Which of the following is not correct ?
Text Solution
|
- The length of the carbon-carbon single bond of the compounds (I)CH(2...
Text Solution
|
- Which of the following involeves no displacement or shifting of electr...
Text Solution
|
- Which of the following exhibit electromeric effect ?
Text Solution
|
- Point out the incorrect statement about resonance.
Text Solution
|
- Resonationg structures of molecules have :
Text Solution
|
- Shifting of electron of a multiple bond under the influence of a reage...
Text Solution
|
- Give the correct order of increasing acidity of the following compoun...
Text Solution
|
- Consider the following benzyl alcohol : Correct order of their K(...
Text Solution
|
- Resonance energy of these compounds will be in the order as :
Text Solution
|