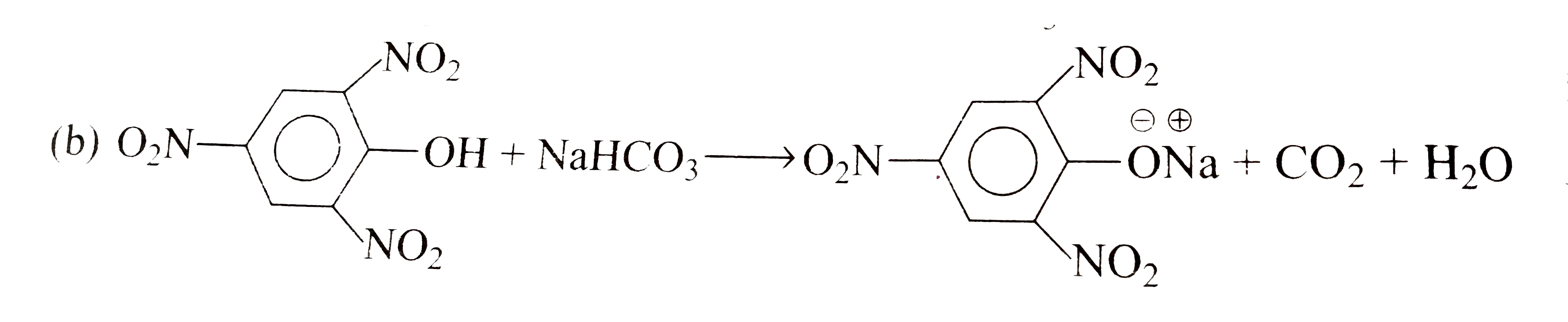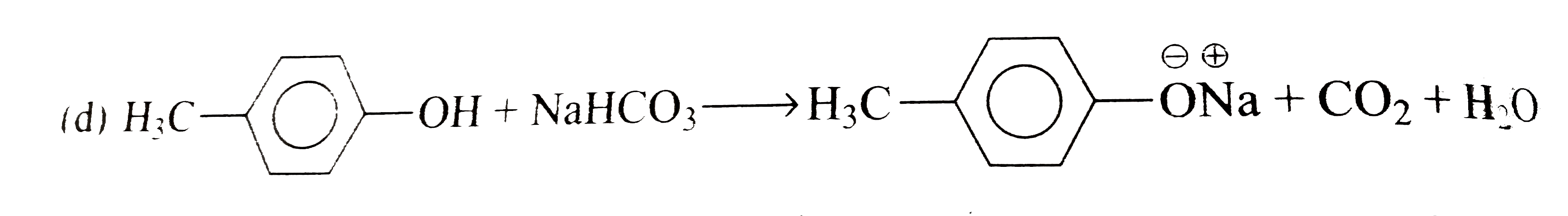A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.101 To Q.125)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.126 To Q.150)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Level 2 (Q.51 To Q.75)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-Level 2 (Q.76 To Q.100)
- Arrange the following compounds in decreasing order of acidity : CH(...
Text Solution
|
- Which one among the following is the least basic?
Text Solution
|
- Which of the following compound cantain most acidic H?
Text Solution
|
- Which of the following reactions does not proceed in forward direction...
Text Solution
|
- Which of the following reactions does not proceed in forward direction...
Text Solution
|
- Which of the following compounds is strogest base?
Text Solution
|
- Give the correct order of decreasing acidity : C(2)H(5)underset((I))...
Text Solution
|
- Which of the following is the strongest base?
Text Solution
|
- Arrange the following compounds in decreasing order of basicity :
Text Solution
|
- Which of the phenol derivative is most acidic?
Text Solution
|
- Arrange the following compounds in increasing order of acidity :
Text Solution
|
- Arrange the following compounds in increasing order of basicity :
Text Solution
|
- rArr The decreasing order of acidity of following phenol derivatives i...
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Arrange in the correct order of acidity
Text Solution
|
- Which is the correct order to stability?
Text Solution
|
- Which is the correct order to stability?
Text Solution
|