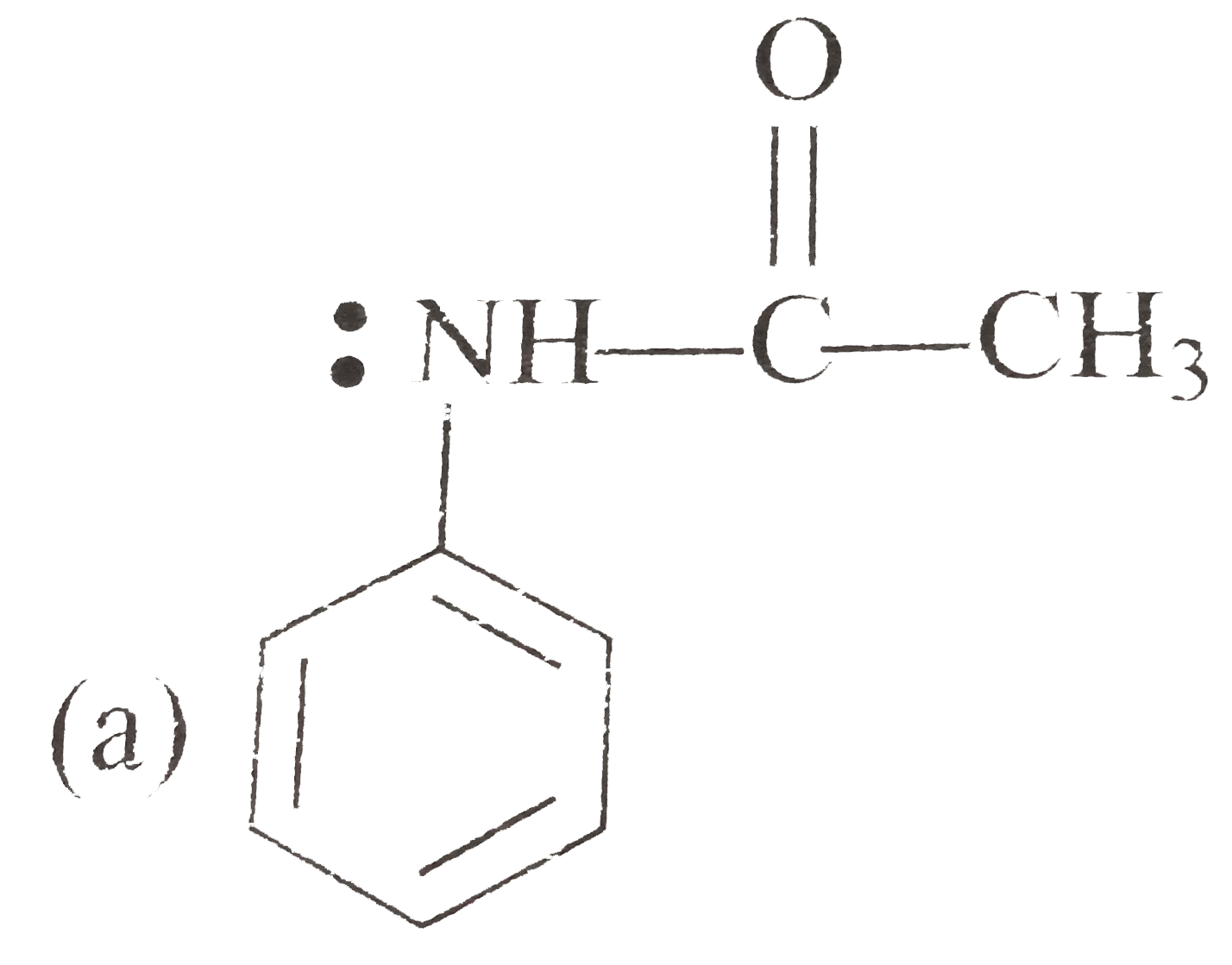
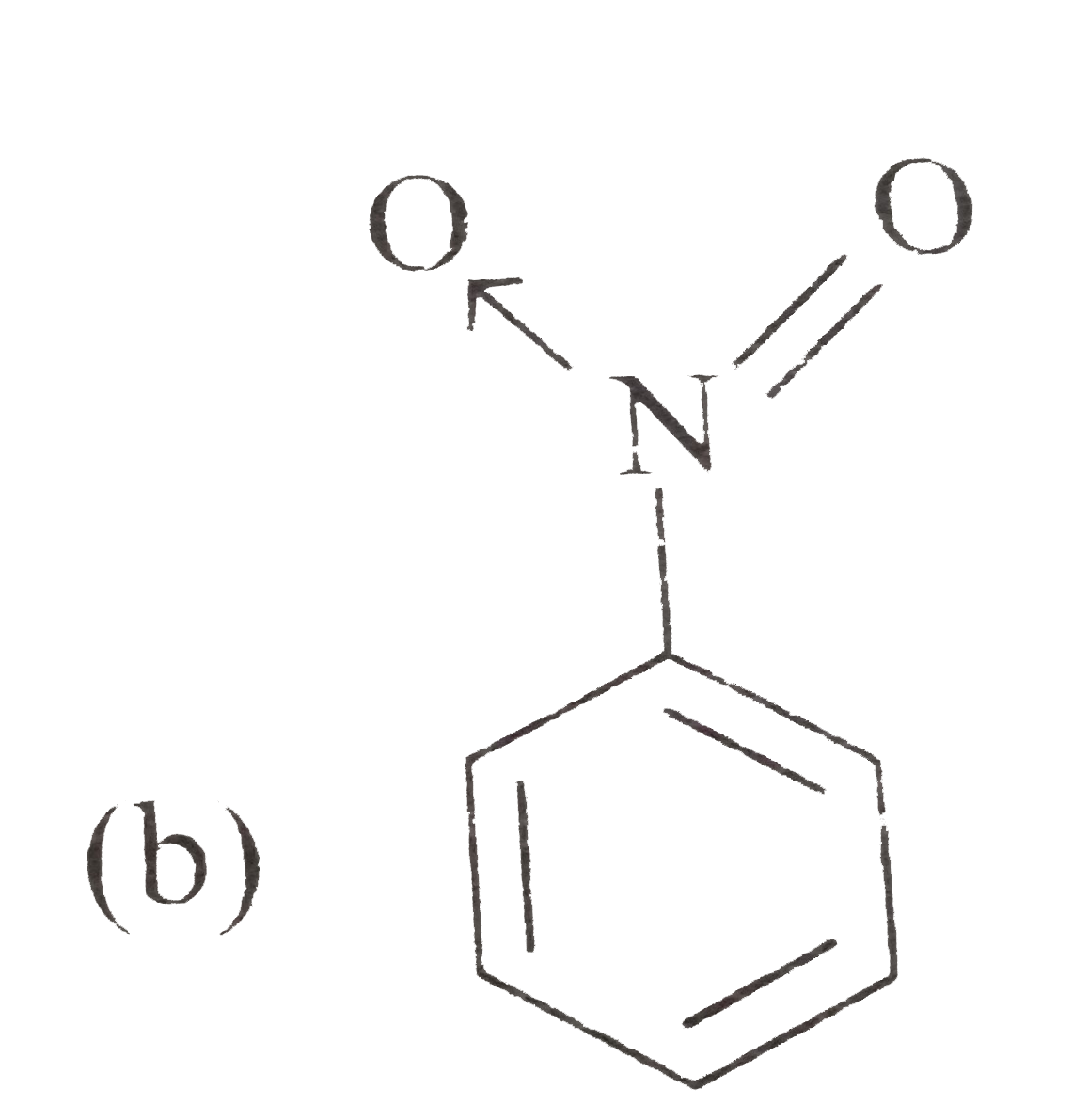
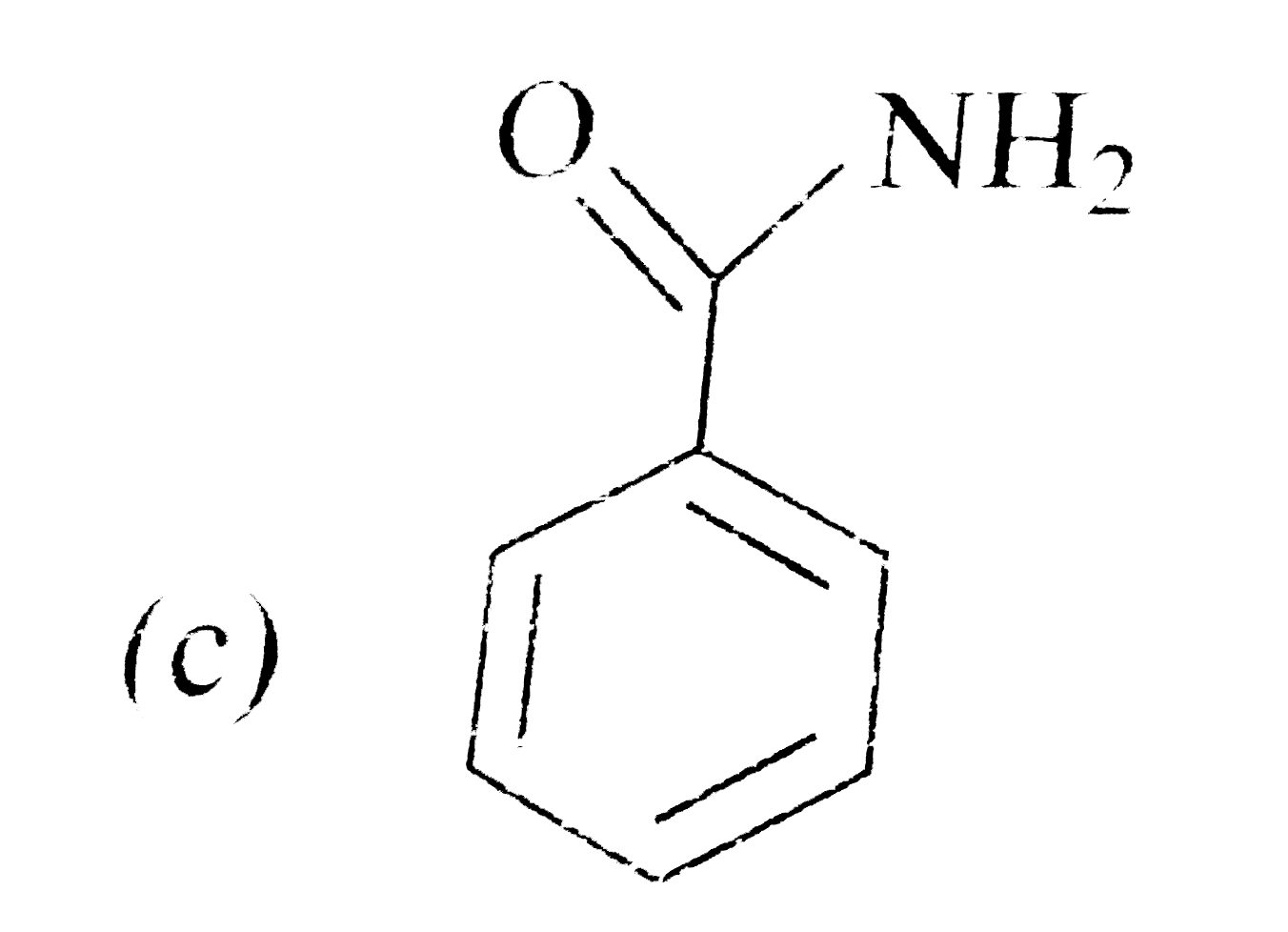
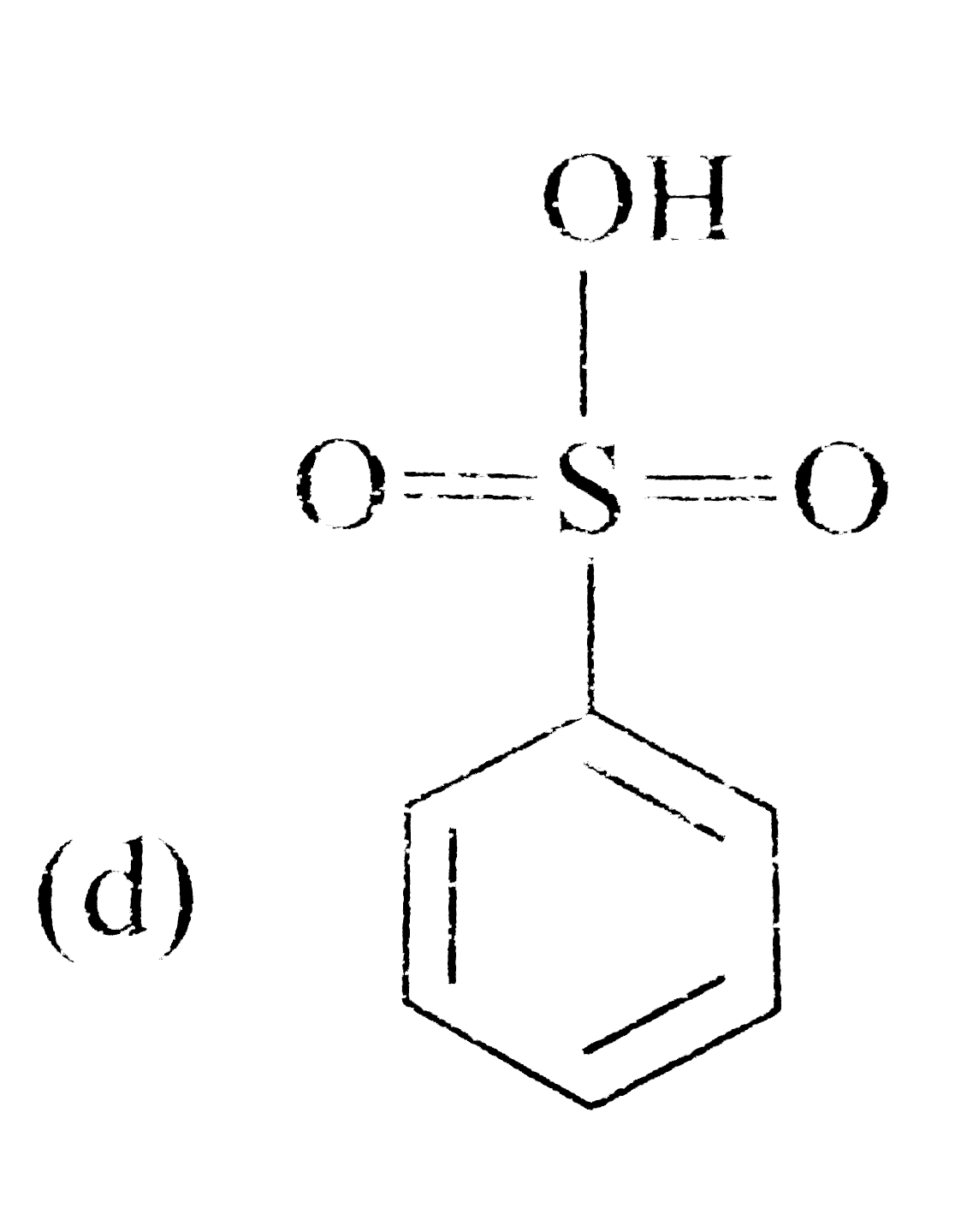
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise More Than One Correct (Q.51 To Q.55)|5 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.1 To Q.25)|25 VideosGENERAL ORGANIC CHEMISTRY
HIMANSHU PANDEY|Exercise More Than One Correct (Q.1 To Q.25)|25 VideosCHEMISTRY IN DAILY LIFE
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|7 VideosHALIDES
HIMANSHU PANDEY|Exercise Subjective Type Problems|10 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-GENERAL ORGANIC CHEMISTRY-More Than One Correct (Q.26 To Q.50)
- Polarisation of electrons in acrolein cannot be written as :
Text Solution
|
- Which among the following statements are correct?
Text Solution
|
- Which among following statements are correct?
Text Solution
|
- Which of these statement are correct?
Text Solution
|
- Which of these statement are correct?
Text Solution
|
- Which of these statement are correct?
Text Solution
|
- Which of the following are nucleophiles?
Text Solution
|
- Which of the following are electrophiles?
Text Solution
|
- Which of the following are less basic than H(2)C=overset(* *)NH?
Text Solution
|
- Which of the following compounds are linear?
Text Solution
|
- Among the following which statement are correct?
Text Solution
|
- Pyridine is more basic than pyrrole. Which of these of following state...
Text Solution
|
- Among the following which statement are correct?
Text Solution
|
- Which of the following statement are correct about this pair of geomet...
Text Solution
|
- In which of the following molecules -NO(2) group is not coplanar with ...
Text Solution
|
- In which of the following molecules both phenyl rings are coplanar?
Text Solution
|
- Find out correct statement regarding resonance energy :
Text Solution
|
- Which of the following statement are incorrect about this molecule?
Text Solution
|
- Which of the following compounds will create electron at ortho and par...
Text Solution
|
- Which of the following compounds have electron deficiency at ortho and...
Text Solution
|