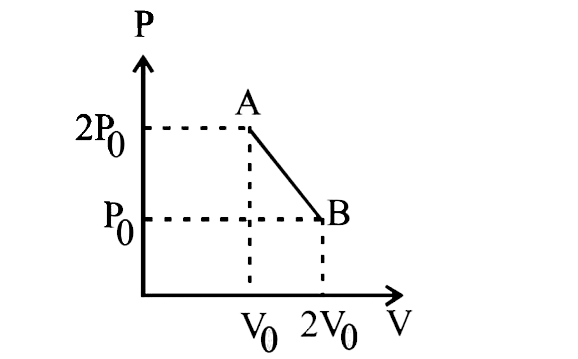
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HEAT-2
MOTION|Exercise EXERCISE-4 (LEVEL-II)|30 VideosHEAT-2
MOTION|Exercise EXERCISE-3 (LEVEL-II)|15 VideosHEAT TRANSFER & THERMAL EXPANSION
MOTION|Exercise Exercise - 3 Section-B|19 VideosHYDROSTATIC, FLUID MECHANICS & VISCOSITY
MOTION|Exercise EXERCISE -3 (SECTION-B) PREVIOUS YEAR PROBLEM|7 Videos
Similar Questions
Explore conceptually related problems
MOTION-HEAT-2 -EXERCISE-4 (LEVEL-I)
- The potential energy function for the force between two atoms in a dia...
Text Solution
|
- Three perfect gases at absolute temperature T(1), T(2) and T(3) are mi...
Text Solution
|
- A carnot engine operating between temperatures T(1) and T(2) has effic...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- A container with insulating walls is divided into two equal parts by a...
Text Solution
|
- The specific heat capacity of a metal at low temperature (T) is given ...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- An ideal gas enclosed in a vertical cylindrical container supports a f...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|
- An open glass tube is immersed in mercury in such a way that a length ...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- Consider an ideal gas confined in an isolated closed chamber. As the g...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- An ideal gas under goes a quasi static, reversible process in which it...
Text Solution
|
- C(p) nad C(v) are specific heats at constant pressure and constant vol...
Text Solution
|
- The temperature of an open room of volume 30 m^(3) increases from 17^(...
Text Solution
|
- Two moles of an ideal monatomic gas occupies a volume V at 27 °C. The ...
Text Solution
|