Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ELECTROCHEMISTRY-Exercise
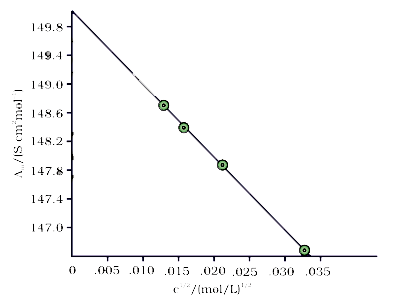
- The molar conductivity of KCl solution at different concentrations at ...
Text Solution
|
- How would you determine the standard reduction potential of the system...
Text Solution
|
- Can you store CuSO(4) solution in Zn pot ?
Text Solution
|
- Consult the table of standard electrode potential and suggest three su...
Text Solution
|
- Calculate the potential of hydrogen electrode in contact with a soluti...
Text Solution
|
- Calculate the EMF of the cell in which the following reaction takes pl...
Text Solution
|
- The cell in which the following reaction occurs 2Fe^(3+)(aq)+2I^(-)(...
Text Solution
|
- Why does the conductivity of a solution decreases with dilution ?
Text Solution
|
- Suggest a way to determine wedge(m^(@)) value of water.
Text Solution
|
- The molar conductivity of 0.25 mol L^(-1) methanoic acid is 46.1 S cm^...
Text Solution
|
- If a current of 0.5A flows through a metallic wire for 2 hours, then h...
Text Solution
|
- Suggest a list of metals that are extracted electrolytically.
Text Solution
|
- Consider the reaction : Cr(2)O(7)^(2-)+14H^(o+)+6e^(-) rarr 2Cr^(+3)...
Text Solution
|
- Write the Chemistry of recharging of lead storage battery highlighting...
Text Solution
|
- Suggest two materials other than hydrogen that can be used as fuels in...
Text Solution
|
- Explain how rusting of iron is envisaged as setting up of an electroch...
Text Solution
|
- Arrange the following metals in the order in which they displace each ...
Text Solution
|
- Given standard electrode potentials K^(o+)|K=-2.93V, Ag^(o+)|Ag=0.80...
Text Solution
|
- Depict the galvanic cell in which the reaction : Zn(s)+2Ag^(o+)(aq) ...
Text Solution
|
- Calculate the standard cell potentials of galvanic cell in which the ...
Text Solution
|
- Write the Nernst equation and EMF of the following cells at 298K : a...
Text Solution
|