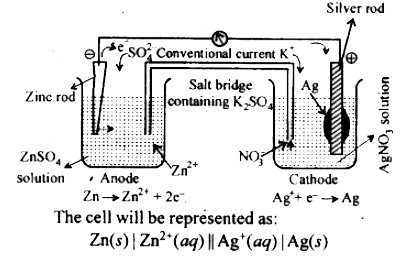
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-ELECTROCHEMISTRY-Exercise
- Suggest two materials other than hydrogen that can be used as fuels in...
Text Solution
|
- Explain how rusting of iron is envisaged as setting up of an electroch...
Text Solution
|
- Arrange the following metals in the order in which they displace each ...
Text Solution
|
- Given standard electrode potentials K^(o+)|K=-2.93V, Ag^(o+)|Ag=0.80...
Text Solution
|
- Depict the galvanic cell in which the reaction : Zn(s)+2Ag^(o+)(aq) ...
Text Solution
|
- Calculate the standard cell potentials of galvanic cell in which the ...
Text Solution
|
- Write the Nernst equation and EMF of the following cells at 298K : a...
Text Solution
|
- In the button cells widely used in watches and other devices the follo...
Text Solution
|
- Define conductivity and molar conductivity for the solution of an ele...
Text Solution
|
- The conductivity of 0.20M solution of KCl at 298K is 0.0248 S cm^(-1) ...
Text Solution
|
- The resistance of a conductivity cell contaning 0.001M KCl solution at...
Text Solution
|
- The conductivity of sodium Chloride at 298K has been determine at di...
Text Solution
|
- The conductivity of 0.00241 M acetic acid is 7.896xx10^(-5)Scm^(-1). C...
Text Solution
|
- How much Charge is required for the following reductions : a. 1 "mo...
Text Solution
|
- How much electricity in terms of Faraday is required to produce. a. ...
Text Solution
|
- How much electricity is required in coulomb for the oxidation of : (...
Text Solution
|
- A solution of Ni(NO(3))(2) is electrolyzed between platium electrodes...
Text Solution
|
- Three electrolytic cell A,B, and C contaning solutions of ZnSO(4), AgN...
Text Solution
|
- Using the standard electrode potentials given in the electrode potent...
Text Solution
|
- Predict the products of electrolysis in each of the following : a. A...
Text Solution
|