Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-HYDROCARBONS-EXERCISE
- An alkene ‘A’ contains three C – C, eight C – H (sigma) bonds and one...
Text Solution
|
- Propanal and pentan-3-one are the ozonolysis products of an alkene? Wh...
Text Solution
|
- Write chemical equations for combustion reaction of the following hydr...
Text Solution
|
- Draw the cis and trans structures of hex-2-ene. Which isomer will have...
Text Solution
|
- Why is benzene extra ordinarily stable though it contains three double...
Text Solution
|
- What are the necessary conditions for any system to be aromatic?
Text Solution
|
- Explain why the following systems are not aromatic?
Text Solution
|
- How will you convert benzene into (i) p-nitrobromobenzene (ii) m-...
Text Solution
|
- In the alkane H3C-CH(2)-C(CH(3))2-CH(2)-CH(CH(3))2, identify 1^(@),2^(...
Text Solution
|
- What effect does branching of an alkane chain has on its boiling point...
Text Solution
|
- Addition of HBr to propene yields 2-bromopropane, while in the presenc...
Text Solution
|
- Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylen...
Text Solution
|
- Arrange benzene, n-hexane and ethyne in decreasing order of acidic beh...
Text Solution
|
- Why does benzene undergo electrophilic substitution reactions easily a...
Text Solution
|
- How would you convert the following compounds into benzene? (i) Ethy...
Text Solution
|
- Write structures of all the alkenes which on hydrogenation give 2-meth...
Text Solution
|
- Arrange the following set of compounds in order of their decreasing re...
Text Solution
|
- Out of benzene, m–dinitrobenzene and toluene which will undergo nitrat...
Text Solution
|
- Suggest the name of a Lewis acid other than anhydrous aluminium chlori...
Text Solution
|
- Why is Wurtz reaction not preferred for the preparation of alkanes con...
Text Solution
|
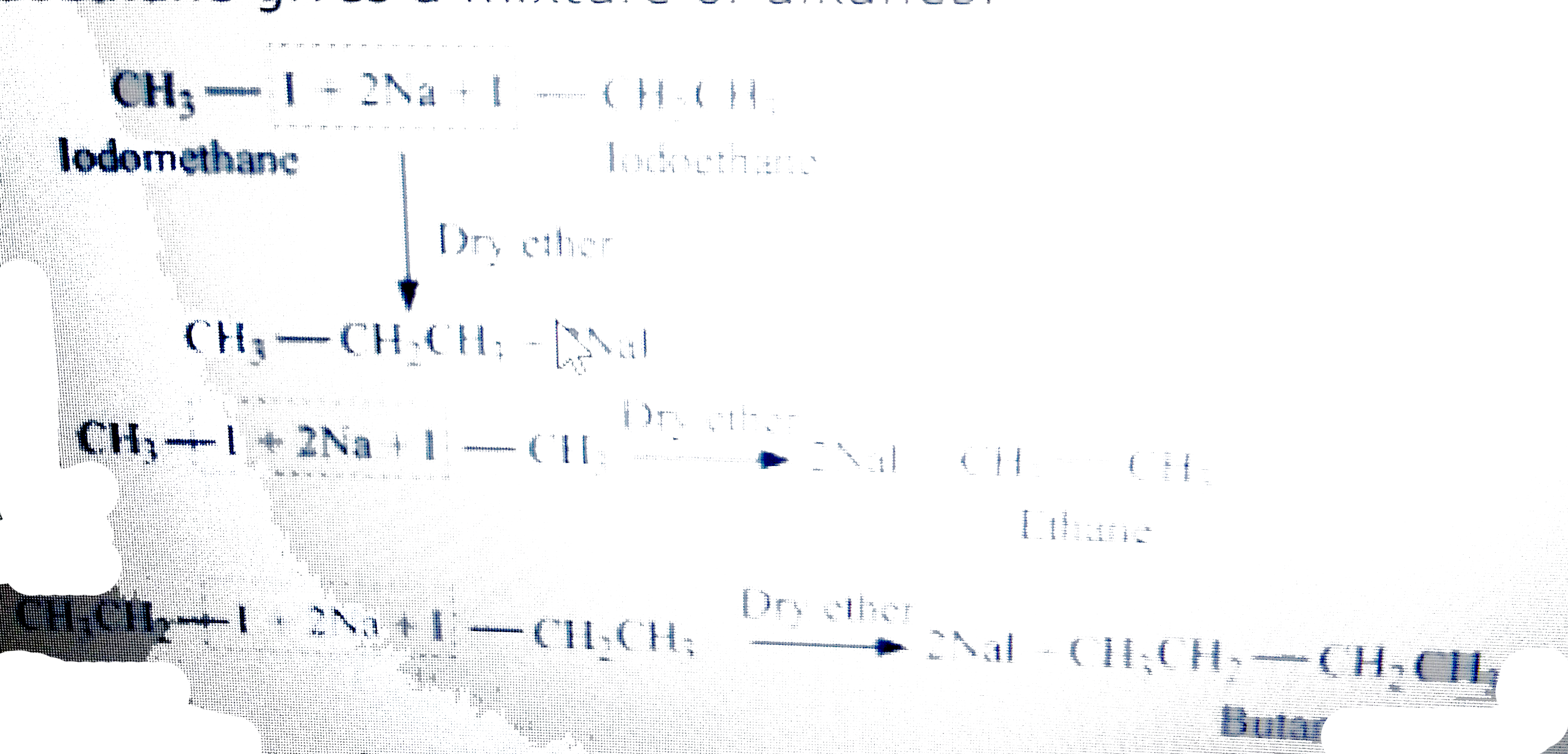 Wurtz reaction cannot be used for the preparation of unsymmetrical alkanes because if two dissimilar alkyl halides are taken as the reactants, then a mixture of alkanes is obtained as the products. Since the reaction involves free radical species, a side reaction also occurs to produce an alkene. For example, the reaction of bromomethane and iodoethane gives a mixture of alkanes.
Wurtz reaction cannot be used for the preparation of unsymmetrical alkanes because if two dissimilar alkyl halides are taken as the reactants, then a mixture of alkanes is obtained as the products. Since the reaction involves free radical species, a side reaction also occurs to produce an alkene. For example, the reaction of bromomethane and iodoethane gives a mixture of alkanes.  The boiling points of alkanes (obtained in the mixture) are very close. Hence, it becomes difficult to separate them.
The boiling points of alkanes (obtained in the mixture) are very close. Hence, it becomes difficult to separate them.