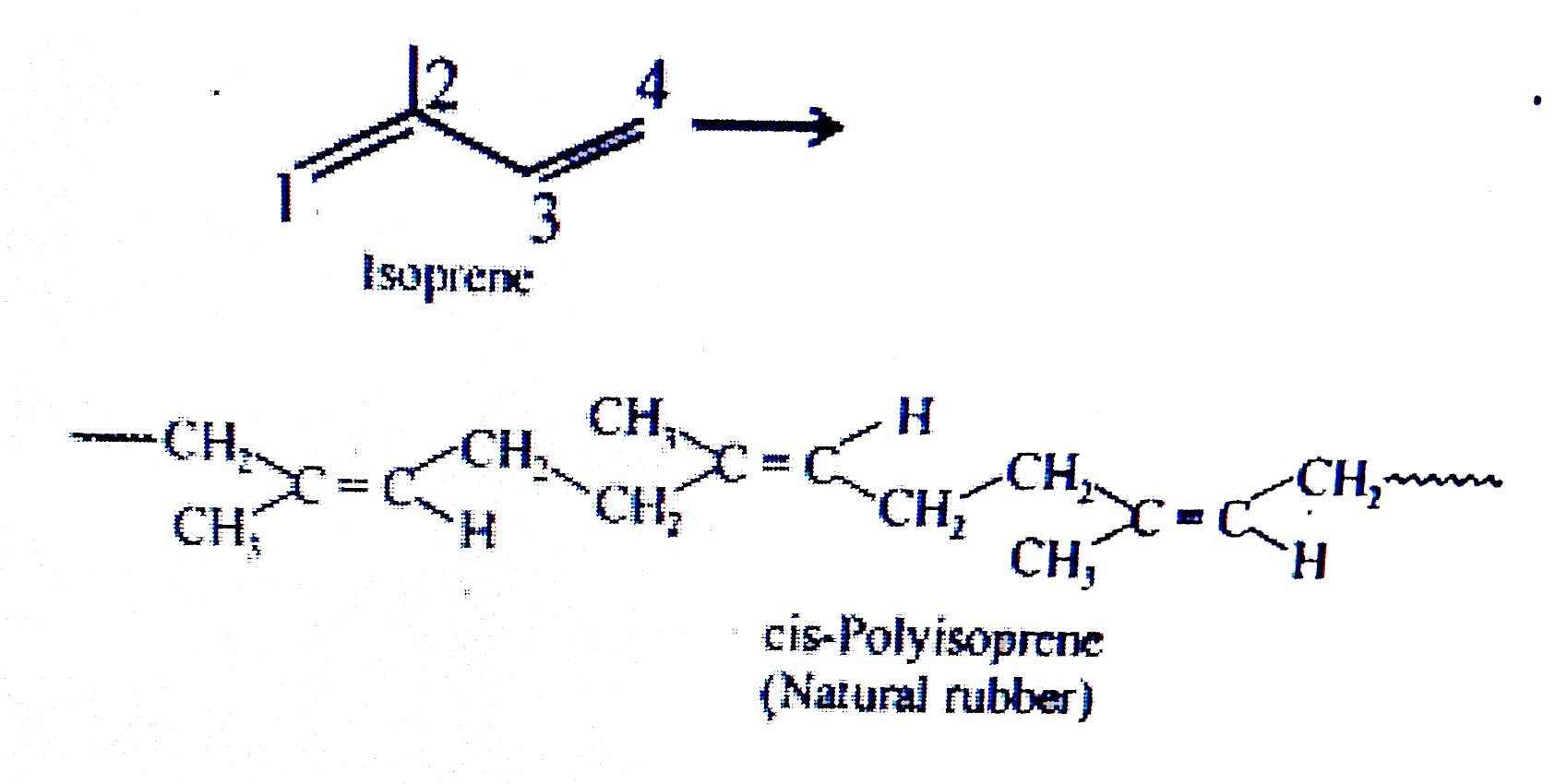
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-POLYMERS-Exercise
- Explain the terms polymer and monomer.
Text Solution
|
- What are natural and synthetic polymers ? Give two examples of each ty...
Text Solution
|
- Distinguish between the terms homopolymer and copolymer and give an ex...
Text Solution
|
- How do you explain the functionality of a monomer?
Text Solution
|
- Define the term polymersation.
Text Solution
|
- Is(NH-CHR-CO)(n)a homopolymer or copolymer?
Text Solution
|
- In which classes, the polymers are classified on the basis of molecula...
Text Solution
|
- How can you differentiate between addition and condensation polymerisa...
Text Solution
|
- Explain the term copolymersation and give two examples.
Text Solution
|
- Write the free radical mechanism for the polymersation of ethene.
Text Solution
|
- Define thermoplastics and thermosetting polymers with two examples of ...
Text Solution
|
- Write the monomers used for getting the following polymers. (i) Poly...
Text Solution
|
- Write the name and structure of one of the common initiators used in f...
Text Solution
|
- How does the presence of double bonds in rubber molecules influence th...
Text Solution
|
- Discuss the main purpose of vulcanisation of rubber.
Text Solution
|
- Which are the monomeric repeating units of Nylon-6 and Nylon-6,6 ?
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- Identify the monomer in the following polymeric structures. (i)
Text Solution
|
- How is Dacron obtained from ethylene glycol and terephthalic acid?
Text Solution
|
- What is a biodegradable polymer ? Give an example of a biodegradable a...
Text Solution
|