Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-KINETIC THEORY-EXERCISE
- Estimate the fraction of molecular volume to the actual volume occupie...
Text Solution
|
- Molar volume is the volume occupied by 1 mole of any (Ideal) gas at st...
Text Solution
|
- Fig shows of PV//T versus P for 1.00 xx 10^(-3) kg of oxygen gas at tw...
Text Solution
|
- An oxygen cylinder of volume 30 litres has an initial gauge pressure o...
Text Solution
|
- An air bubble of volume 1.0 cm^(3) rises from the bottom of a lake 40 ...
Text Solution
|
- Estimate the total number of air molecules (inclusive of oxygen, nitro...
Text Solution
|
- Estimate the average thermal energy of a helium atom at (i) room tempe...
Text Solution
|
- Three vessel of equal capacity have gases at the same temperature and ...
Text Solution
|
- At what temperature is the root mean square speed of an atom in an arg...
Text Solution
|
- Estimate the mean free path and collision frequency of a nitrogen mole...
Text Solution
|
- A metre long narrow bore held horizontally (and close at one end) cont...
Text Solution
|
- From a certain apparatus, the diffusion rate of hydrogen has an averag...
Text Solution
|
- A gas in equilibrium has uniform density and pressure throughout its v...
Text Solution
|
- Given below are densities of some solids and liquids. Give rough estim...
Text Solution
|
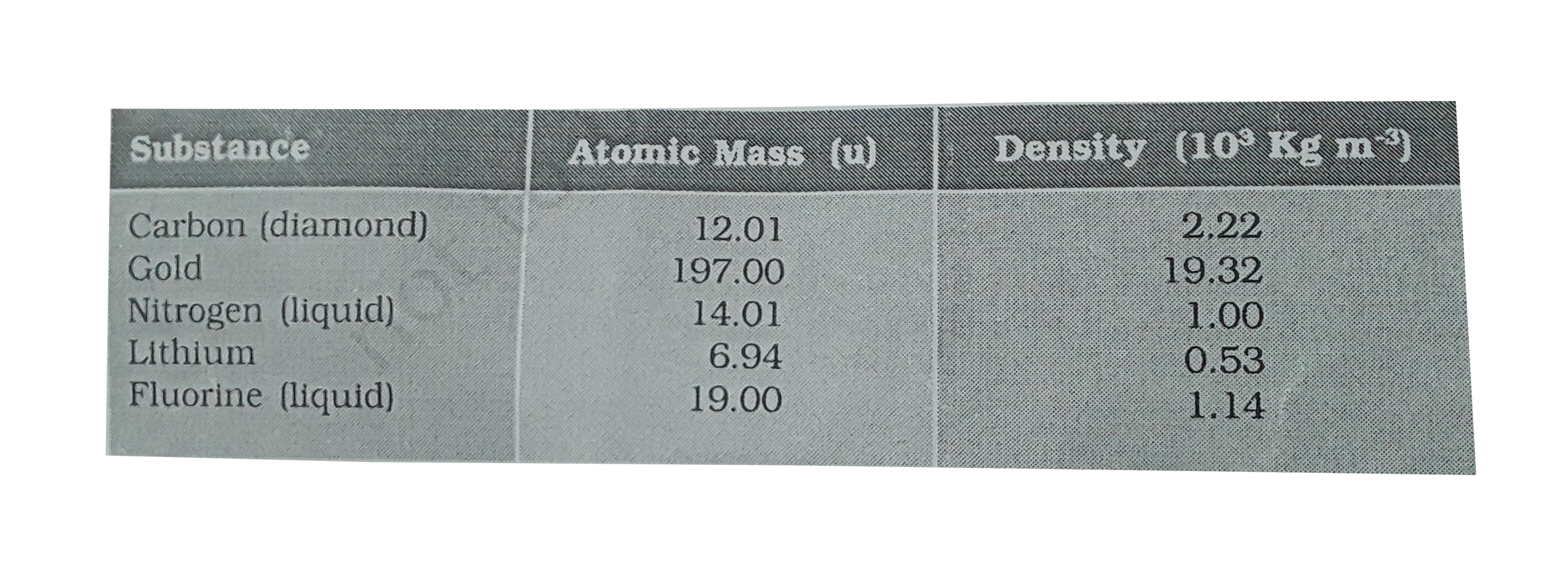 [Hint : Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro’s number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å ].
[Hint : Assume the atoms to be ‘tightly packed’ in a solid or liquid phase, and use the known value of Avogadro’s number. You should, however, not take the actual numbers you obtain for various atomic sizes too literally. Because of the crudeness of the tight packing approximation, the results only indicate that atomic sizes are in the range of a few Å ].