Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT-COORDINATION COMPOUNDS-Exercise
- Using IUPAC norms write the fomulas for the following (i). Tetrahydr...
Text Solution
|
- Using IUPAC norms write the systematic names of the following: (i). ...
Text Solution
|
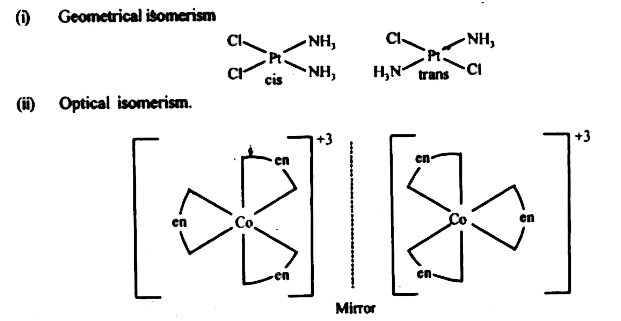
- List various types of isomerism possible for coordination compounds, g...
Text Solution
|
- How many geometrical isomers are possible in the following corrdinatio...
Text Solution
|
- Draw the structures of optical isomers of: (i). [Cr(C2O4)3]^(3-) (...
Text Solution
|
- Draw all the isomers (geometrical and optical) of: (i) [CoCl(2)(en)(...
Text Solution
|
- Write all the geometrical isomers of [Pt(NH3)(Br)(Cl)(py)] and how man...
Text Solution
|
- Aqueous copper sulphate solution (blue in colour) gives: (i). A gree...
Text Solution
|
- What is the coordination entity formed when excess of aqueous KCN is a...
Text Solution
|
- Discuss the nature of bonding in the following coordination entities o...
Text Solution
|
- Draw figure to show the splitting of d orbitals in an octahedral cryst...
Text Solution
|
- What is spectrochemical series? Explain the difference between a weak ...
Text Solution
|
- What is crystal field splitting energy? How does the magnitude of tria...
Text Solution
|
- [Cr(NH3)6]^(3+) is paramagnetic while [Ni(CN)4]^(2-) is diamagnetic. E...
Text Solution
|
- A solution of [Ni(H(2)O)(6)]^(2+) is green but a solution of [Ni(CN)(4...
Text Solution
|
- [Fe(CN)(6)]^(4-) and [Fe(H(2)O)(6)]^(2+) are of different colours in d...
Text Solution
|
- BONDING IN METAL CARBONYLS
Text Solution
|
- Give the oxidation state, d-orbitals occupation and coordination numbe...
Text Solution
|
- Wrtie down the IUPAC name for each of the following complexes and indi...
Text Solution
|
- Explain the violet colour of the complex [Ti(H(2)O)(6)]^(3+) on the ba...
Text Solution
|