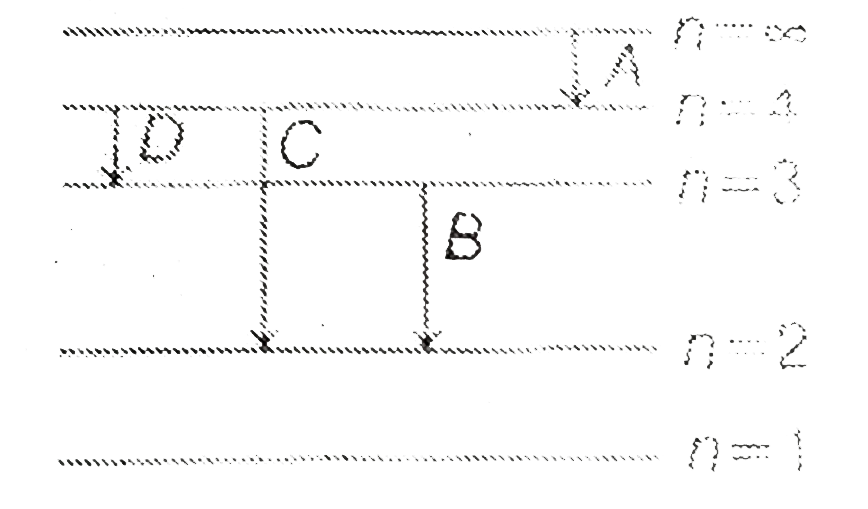A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
MODERN PHYSICS -1
MOTION|Exercise EXERCISE-2(LEVEL-I)|34 VideosMODERN PHYSICS -1
MOTION|Exercise EXERCISE-2(LEVEL-II)|15 VideosMODERN PHYSICS -1
MOTION|Exercise EXERCISE-4 ( LEVEL- II)|39 VideosMODERN PHYSICS - 2
MOTION|Exercise EXERCISE - 4 (Level -II) PREVIOUS YEAR - JEE ADVANCED|28 VideosNEWTON'S LAWS OF MOTION & FRICTION
MOTION|Exercise Exercise - 3 Section-B|12 Videos
Similar Questions
Explore conceptually related problems
MOTION-MODERN PHYSICS -1-EXERCISE-1
- Light of wavelength lamda strikes a photoelectric surface and electron...
Text Solution
|
- The angular momentum of an electron in the hydrogen atom is (3h)/(2pi)...
Text Solution
|
- Consider the electron energy level diagram of H-atom Photons associate...
Text Solution
|
- If the electron in a hydrogen atom were in the energy level with n 3,...
Text Solution
|
- In a hydrogen atom, the electron is in n^(th) excited state. It may co...
Text Solution
|
- Difference between n^(th) and (n+1)^(th) Bohr's radius of H atom is eq...
Text Solution
|
- The electron in a hydrogen atom makes a transition from M shell to L-s...
Text Solution
|
- The electron in a hydrogen atom makes a transition n(1) rarr n(2) wher...
Text Solution
|
- The radius of Bohr's first orbit is a(0) . The electron in n^(th) orbi...
Text Solution
|
- The ionisation potential of H-atom is 13.6 eV. The energy required to ...
Text Solution
|
- In a sample of hydrogen-like atom all of which are in ground state, a ...
Text Solution
|
- A neutron collies head-on with a stationary hydrogen atom in ground st...
Text Solution
|
- The electron in a hydrogen atom make a transition from an excited stat...
Text Solution
|
- An H atom in ground state is moving with initial kinetic energy K .It ...
Text Solution
|
- An electron collides with a hydrogen atom in its ground state and exci...
Text Solution
|
- An electron with kinetic energy 5eV is incident on a hydrogen atom in ...
Text Solution
|
- An electron of energy 11.2 eV undergoes an inelastic collision with a...
Text Solution
|
- The recoil speed of hydrogen atom after it emits a photon in going fro...
Text Solution
|
- The recoil speed of a hydrogen atom after it emits a photon is going...
Text Solution
|
- A neutron moving with speed a makes a heat on collision with a hydroge...
Text Solution
|