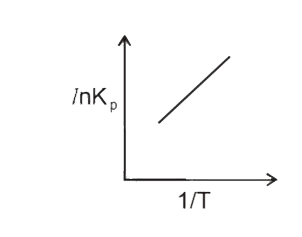
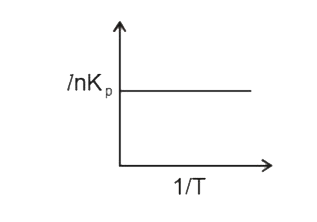
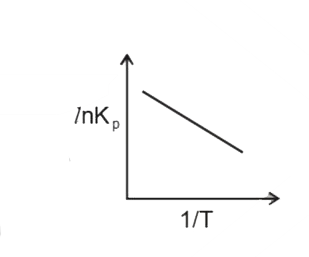
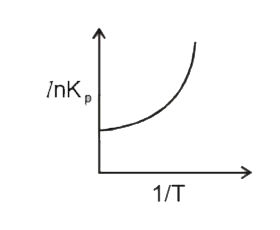
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 2|99 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 3|67 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise SOLVED EXAMPLES|10 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)|1 Videos
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL EQUILIBRIUM-Exercise - 1
- Which of the following relative values of k(f) and k(b) results in an ...
Text Solution
|
- The role of a catalyst in a reversble reaction is to
Text Solution
|
- An exothermic reaction is represented by the greph :
Text Solution
|
- Variation of log(10)K with (1)/(T) is shown by the following graph in ...
Text Solution
|
- Listed in the table are forward and reverse rate constants for the rea...
Text Solution
|
- Consider the reaction CaCO(3)(s) hArr CaO(s) +CO(2)(g) in closed c...
Text Solution
|
- In the melting of ice, which one of the conditions will be more favour...
Text Solution
|
- In the reaction, 2SO(2)(s)+O(2)(g) hArr 2SO(3)(g)+X cal, most favourab...
Text Solution
|
- On adding inert gas to the equilibrium PCI(5)(g)hArrPCI(3)(g) + CI(2)(...
Text Solution
|
- Dissociation of phosphorus pentachloride is favoured by -
Text Solution
|
- Adding inert gas to system N(2(g)) + 3H(2(g))hArr2NH(3(g)) at equilibr...
Text Solution
|
- In the reaction N(2(g)) + 3H(2(g))hArr2NH(3(g)), the forward reaction ...
Text Solution
|
- The reaction in which the yield of the products can not be increased b...
Text Solution
|
- In the reaction A (g) + B (g)hArr C (g), the backward reaction is favo...
Text Solution
|
- Which among the following conditions, increase the yield of the produc...
Text Solution
|
- When H(2) is added to an equilibrium mixture 2HI(g)hArrH(2(g)) + I(2(g...
Text Solution
|
- For the reaction PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) the forward reactio...
Text Solution
|
- Which of the following reaction will be favoured at low pressure?
Text Solution
|
- The what manner will increase of pressure affect the following equati...
Text Solution
|
- For the reaction PCl(5)(g)toPCl(3)(g)+Cl(2)(g)
Text Solution
|