A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 3|67 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 1|45 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)|1 Videos
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL EQUILIBRIUM-Exercise - 2
- At 675 K, H(2)(g) and CO(2)(g) react to form CO(g) and H(2)O(g), K(p) ...
Text Solution
|
- 2 Moles each of SO3,CO,SO2 and CO2 is taken in a one lit. vessel. If K...
Text Solution
|
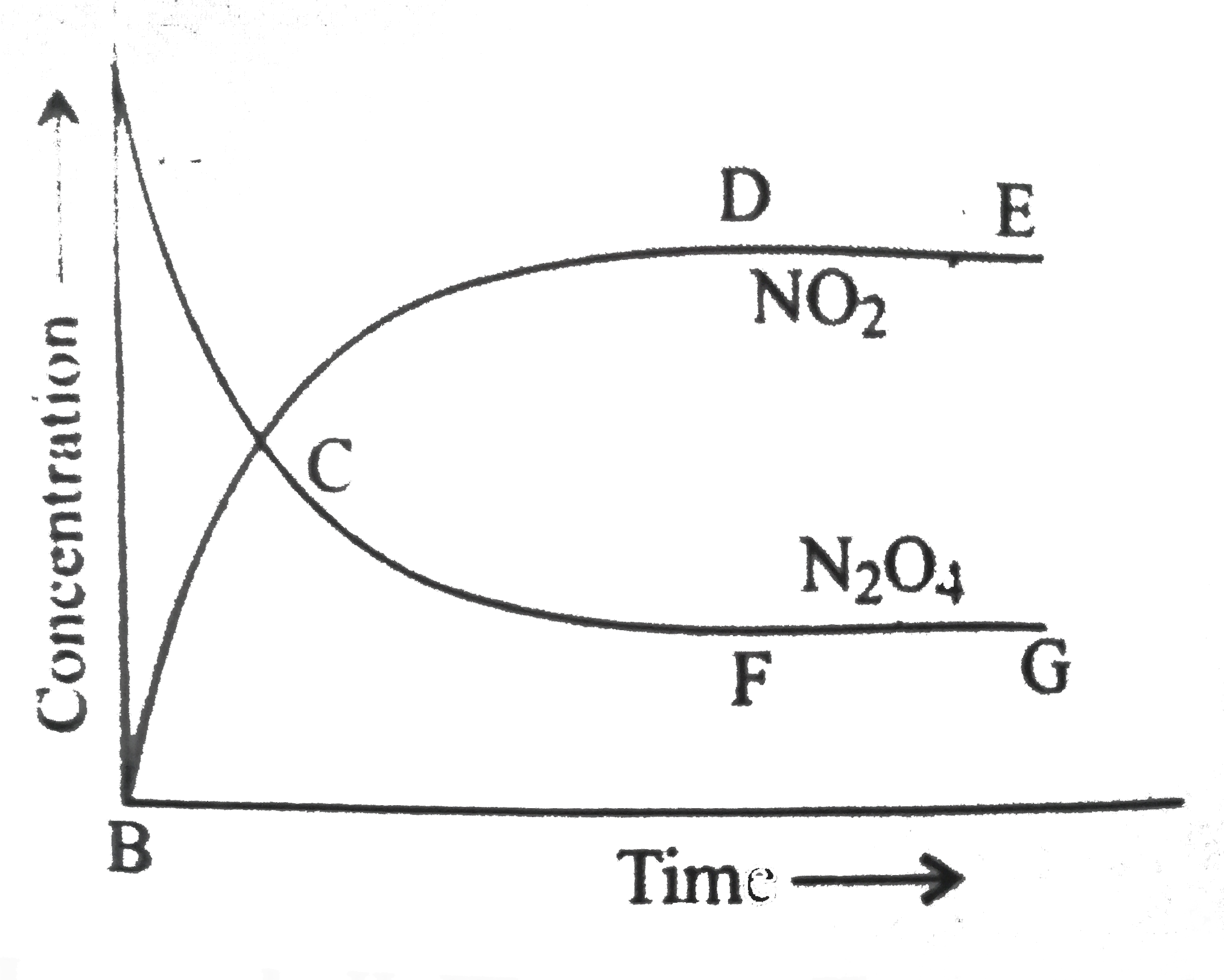
- N(2)O(4) hArr 2NO(2), K(c)=4. This reversible reaction is studied grap...
Text Solution
|
- For the reaction H(2)(g) + I(2)(g)hArr2HI(g)K(c) = 66.9 at 350^(@)C an...
Text Solution
|
- The equilibrium constant for, 2H(2)S (g)hArr 2H(2)(g) + S(2)(g) is 0.0...
Text Solution
|
- For a reversible reaction aA + bBhArrcC + dD , the variation of K with...
Text Solution
|
- In which of the following reactions, increase in the pressure at const...
Text Solution
|
- Changing the volume of the system does not after the number of moles i...
Text Solution
|
- The conditions favourable for the reaction : 2SO(2)(g)+O(2)(g) 2SO(3...
Text Solution
|
- The exothermic formation of ClF(3) is represented by thr equation: C...
Text Solution
|
- The equilibrium SO(2)Cl(2)(g) hArr SO(2)(g)+Cl(2)(g) is attained at 25...
Text Solution
|
- For the reaction : PCl(5) (g) rarrPCl(3) (g) +Cl(2)(g):
Text Solution
|
- The yield of product in the reaction, A(2)(g)+2B(g) hArr C(g)+Q KJ ...
Text Solution
|
- What is the effect of the reduction of the volume of the system for th...
Text Solution
|
- Two solid A and B are present in two different container having same v...
Text Solution
|
- Solid A and B are taken in a closed container at a certain temperature...
Text Solution
|
- For the equilibrium CuSO(4)xx5H(2)O(s)hArrCuSO(4)xx3H(2)O(s) + 2H(2)O(...
Text Solution
|
- For the equilibrium, SrCl(2).6H(2)O(s) hArr SrCl(2).2H(2)O(s)+4H(2)O(...
Text Solution
|
- Which of the following statements is (are) correct ?
Text Solution
|
- The value of equilibrium constant of a reversible reaction at a given ...
Text Solution
|