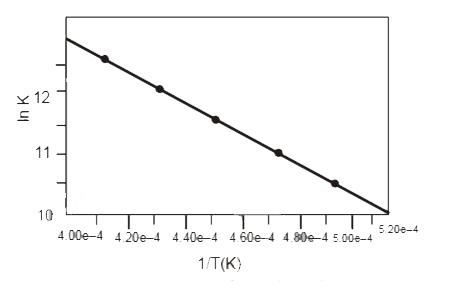
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 3|67 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 1|45 VideosCHEMICAL BONDING
MOTION|Exercise EXERCISE -4 LEVEL-II|40 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)|1 Videos
Similar Questions
Explore conceptually related problems
MOTION-CHEMICAL EQUILIBRIUM-Exercise - 2
- For the gas phase exothermic reaction. A(2) + B(2) hArrC(2), carried...
Text Solution
|
- Consider the equilibrium HgO(s)+4I^(-)(aq)+H(2)O(l)hArrHg I(4)^(2-)(...
Text Solution
|
- In the laboratory the equilibrium constant for a particular reaction c...
Text Solution
|
- Decrease in the pressure for the following equilibria : H(2)O(s)hArr...
Text Solution
|
- Assertion: Water boils at higher temperature in pressure cooker. Rea...
Text Solution
|
- Statement-1 : A decrease in pressure leads to an increase in freezing ...
Text Solution
|
- Assertion: The solubility of gases always increases with increase in p...
Text Solution
|
- Statement-1 : Total number of moles in a closed system at new equilibr...
Text Solution
|
- Statement-1 : An exothermic reaction, nonspontaneous at high temperatu...
Text Solution
|
- Statement-1 : Ammonia at a pressure of 10 atm and CO(2) at a pressure ...
Text Solution
|
- In a 7.0 L evacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at...
Text Solution
|
- In a 7.0 L evacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at...
Text Solution
|
- In a 7.0 L evacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at...
Text Solution
|
- In a 7.0 L evacuated chamber, 0.50 mol H(2) and 0.50 mol I(2) react at...
Text Solution
|
- Equilibrium constants are given (in atm) for the following reactions a...
Text Solution
|
- Equilibrium constants are given (in atm) for the following reactions a...
Text Solution
|
- Equilibrium constants are given (in atm) for the following reactions a...
Text Solution
|
- If we know the equilibrium constant for a particular reaction, we can ...
Text Solution
|
- If we know the equilibrium constant for a particular reaction, we can ...
Text Solution
|
- If we know the equilibrium constant for a particular reaction, we can ...
Text Solution
|