Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-METALLURGY-EXERCISE 4 (LEVEL - II)
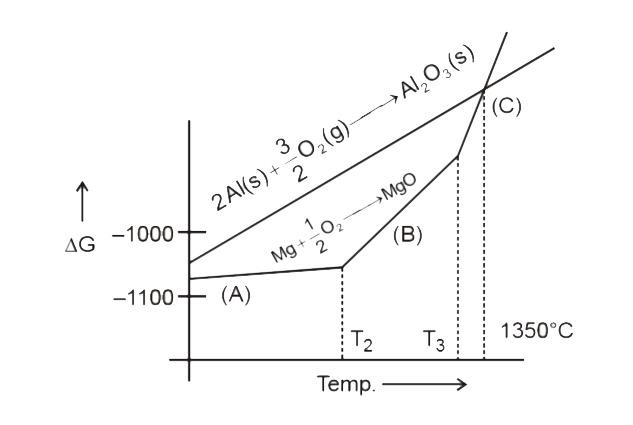
- Which of the following statements are true : A to Mg(s) + 1/2 O2 (g...
Text Solution
|
- Match the extraction processes listed in Column I with metals listed i...
Text Solution
|
- Extraction for zinc from zinc blende is achieved by :
Text Solution
|
- Native silver metal forms a water soluble complex with a dilute aqueou...
Text Solution
|
- Match the conversions in Column I with the type(s) of reaction(s) give...
Text Solution
|
- Copper is the most noble of the first row transition metals and occurs...
Text Solution
|
- Copper is the most noble of the first row transition metals and occurs...
Text Solution
|
- Copper is the most noble of the first row transition metals and occurs...
Text Solution
|
- Oxidation states of the metal in the minerals haematite and magnetite,...
Text Solution
|
- Extraction of metal from the ore cassiterite involves
Text Solution
|
- In the cyanide extraction process of silver from argentite ore, the ox...
Text Solution
|
- Sulphide ores are common for the metals
Text Solution
|
- Upon heating with Cu2S , the reagent(s) that give copper metal is/are
Text Solution
|
- Match the anionic species given in column I that are present in the or...
Text Solution
|
- Copper is purified by electrolytic refining of bliter copper .The curr...
Text Solution
|
- Extraction of copper from copper pyrite (CuFeS(2)) involves
Text Solution
|
- For a reaction taking place in a container in equilibrium with its sur...
Text Solution
|
- Galena (an ore) is partially oxidized by passing air through it at hig...
Text Solution
|