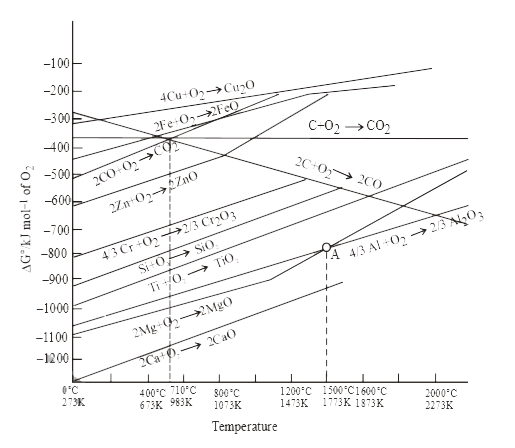
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
METALLURGY
MOTION|Exercise EXERCISE 2 (LEVEL - I)|70 VideosMETALLURGY
MOTION|Exercise EXERCISE 2 (LEVEL - II)|37 VideosMETALLURGY
MOTION|Exercise EXERCISE 4 (LEVEL - II)|17 VideosLIQUID SOLUTION
MOTION|Exercise EXERCISE-4 (Level-II) PREVIOUS YEAR JEE ADVANCED|19 VideosMOLE CONCEPT
MOTION|Exercise EXERCISE - 4 LEVEL - II|13 Videos
Similar Questions
Explore conceptually related problems
MOTION-METALLURGY-EXERCISE 1
- Which of the following curves assures that the metal obtained by carbo...
Text Solution
|
- Which of the following activities is not related to poling ?
Text Solution
|
- The purest variety of iron is called
Text Solution
|
- The Elingham diagram (Fig.) represents the formation of oxides of seve...
Text Solution
|
- The Elingham diagram (Fig.) represents the formation of oxides of seve...
Text Solution
|
- The slag obtained during the extraction of copper from coper pyrites i...
Text Solution
|
- A reaction showing slag formation is .
Text Solution
|
- In the metallurgy of iron, when limestone is added to the blast furnac...
Text Solution
|
- A flux is often added to remove impurities from a concentrated ore. ...
Text Solution
|
- Tin and lead can be refined by
Text Solution
|
- Refining of silver is done by :
Text Solution
|
- CO on passing over heated nickel gives
Text Solution
|
- (xiii) Which process represents the change, Ti + 2I(2) rarr TiI(4) r...
Text Solution
|
- Purification of Silicon element used in semiconductors is done by .
Text Solution
|
- Mond's process is used for
Text Solution
|
- In the Hoope’s process for refining of aluminium, the fused materials ...
Text Solution
|
- The oxidation numbers of Fe and S in iron pyrites are
Text Solution
|
- Extraction of zinc from zinc blende is achieved by:
Text Solution
|
- Oxidation states of the metal in the minerals haematite and magnetite,...
Text Solution
|
- In the extraction of nickel of Mond's process, the metal is obtained b...
Text Solution
|