A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GASEOUS STATE
MOTION|Exercise EXERCISE-2(LEVEL-II)|35 VideosGASEOUS STATE
MOTION|Exercise EXERCISE-3|55 VideosGASEOUS STATE
MOTION|Exercise EXERCISE -1 (INTRODUCTION & STATE PARAMETERS)|44 VideosELECTROCHEMISTRY
MOTION|Exercise EXERCISE-4,II|44 VideosGOC
MOTION|Exercise Exercise - 4 Level - II|14 Videos
Similar Questions
Explore conceptually related problems
MOTION-GASEOUS STATE -EXERCISE-2(LEVEL-I)
- The rates of diffusion of SO(3),CO(2),PCl(3) and SO(2) are in the foll...
Text Solution
|
- 20L of SO(2) diffuse through a porous partition in 60 seconds. Volume ...
Text Solution
|
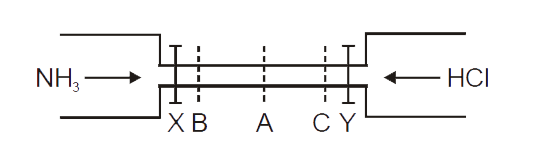
- See the figure : The valves of X and Y opened simulataneously. Th...
Text Solution
|
- X mL of H(2) gas effuses through a hole in a container in 5 s. The tim...
Text Solution
|
- At what temperature will the total KE of 0.3 mol of He be the same as ...
Text Solution
|
- By how many folds the temperature of a gas would increase when the r.m...
Text Solution
|
- The root mean square velocity of an ideal gas in a closed container of...
Text Solution
|
- The temperature of an ideal gas is increased from 140 K to 560 K. If a...
Text Solution
|
- Which of the following gas molecules has the longest mean-free path ?
Text Solution
|
- Temperature at which r.m.s speed of O(2) is equal to that of neon at 3...
Text Solution
|
- The R.M.S. Speed of the molecules of a gas of density kg m^(-3) and p...
Text Solution
|
- The mass of molecule A is twice that of molecule B.The root mean squar...
Text Solution
|
- The temperature of an ideal gas is increased from 120 K to 480 K. If a...
Text Solution
|
- The Van der Waal's parameters for gases W,X,Y and Z are- Which on...
Text Solution
|
- The correct relationship between T(C ) , T(B) and T(i) is -
Text Solution
|
- A real gas obeying van der Waal equation will resemble ideal gas if th...
Text Solution
|
- For the non-zero value of the force of attraction between gas molecule...
Text Solution
|
- Compressibility factor for H(2) behaving as real gas is:
Text Solution
|
- At low pressures, the van der Waal's equation is written as [P + (a)...
Text Solution
|
- In van der Waal's equation of state for a non ideal gas the term that ...
Text Solution
|