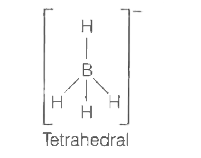
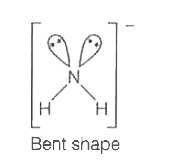
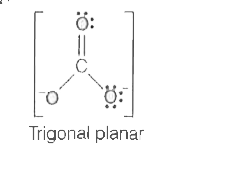
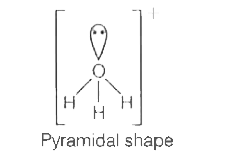
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2019 (3 MAY SHIFT 2)-CHEMISTRY
- The correct increasing order of basic character of Ce (OH)3 ,Gd (OH) 3...
Text Solution
|
- How many ions of the following have bond order of 2.5 ? N2,NO^-,C2^-...
Text Solution
|
- Which of the following ions has tetrahedral geometry and sp^3 - hybrid...
Text Solution
|
- Diffusion of CH4 (g) and O2 (g) occurs under similar conditions, then...
Text Solution
|
- The variation of compressibility factor (Z) with pressure (p in bar) ...
Text Solution
|
- What is the equivalent weight of methanol if one mole of CH3 OH is com...
Text Solution
|
- While combusting in air , 4g of H2 was completely converted into water...
Text Solution
|
- Which of the following does not follow first law of thermodynamics ? (...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- A solution of 0.1 mole of CH3 NH2 ( Kb =5 xx 10^(-4)) and 0.08 mole o...
Text Solution
|
- What are two types of crystal structures shown by ice at different pr...
Text Solution
|
- Identify X and Y respectively in the following reactions
Text Solution
|
- Which one among the following statements is correct about a solution o...
Text Solution
|
- Identify the correct statements from the following : I . Quartz is a...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- Find the suitable IUPAC name of the compound given below underset(OH...
Text Solution
|
- The boiling poing (in K) of cis but -2- ene and dipole moment ( in D) ...
Text Solution
|
- The major product formed in the following reaction sequence is
Text Solution
|
- NaCl is fcc lattice , where Na^+ ions are at corner and face centre po...
Text Solution
|
- How many grams of glucose are required to prepare an aqueous solution ...
Text Solution
|