A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2019 (4 MAY SHIFT 2)-CHEMISTRY
- The order of stability of aromatic hydrocarbons given below is
Text Solution
|
- In a compound AB, A atoms occupy the corners of the cube and the cube ...
Text Solution
|
- Two compounds form an ideal solution at room temperature. Which of the...
Text Solution
|
- If a solute associates in a solvent, its experimentally calculated mol...
Text Solution
|
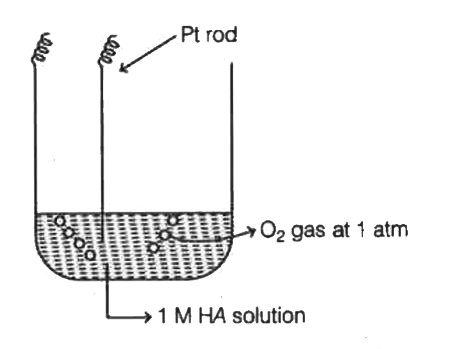
- For a hald cell containing a Pt rod immersed in a solution of 1M HA, O...
Text Solution
|
- When the temperature of a reaction is raised by 10^@C , how many times...
Text Solution
|
- The most effective coagulating agent among the following for Sb2S3 sol...
Text Solution
|
- Which of the following statements are correct related to metallurgy? ...
Text Solution
|
- Identify all the products formed when XeF4 is completely hydrolysed.
Text Solution
|
- What are the compounds formed when white Phosphorous is dissolved in b...
Text Solution
|
- Match the following : The correct answer is
Text Solution
|
- The correct order of the increasing magnetic moments for the followin...
Text Solution
|
- The monomer units of Nylon 6,6 Nylon 2-Nylon 6 are respectively,
Text Solution
|
- The product (s) formed when glucose rreacts with a strong oxidising ag...
Text Solution
|
- Which of the following statements are true foe saccharin. (A) It is ...
Text Solution
|
- The final product "B" of the below reaction sequence is CH3 - overs...
Text Solution
|
- Arrange the following compounds in the correct order of their acid str...
Text Solution
|
- The prouduct (P) of below reactin sequence is CH(3)CH(2) CHO overset...
Text Solution
|
- What are the suitable conditions for the following transformation ?
Text Solution
|
- The compound 'A' decolourises Br2 // "CCl"4 and releases N2 gas with ...
Text Solution
|