A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2017-CHEMISTRY
- Which of the following conditions are correct for real solutions showi...
Text Solution
|
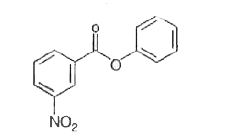
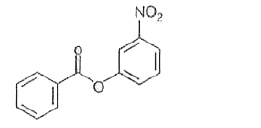
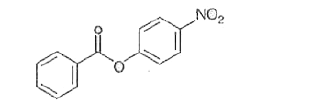
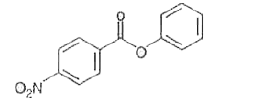
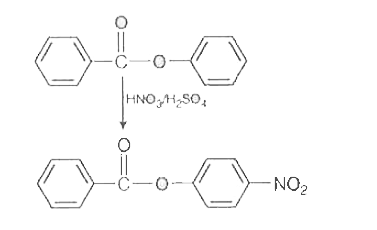
- Nitration of phenyl benzoate yields the product
Text Solution
|
- The electronic configuration of Pr59(praseodimium) is
Text Solution
|
- Which of the following is the most basic oxide?
Text Solution
|
- The element that forms stable compunds in low oxidation state is
Text Solution
|
- Atomic radius (pm) of Al, Si, N and F respectively is
Text Solution
|
- Reaction of calgon with hard water containing Ca^2+ ions produce
Text Solution
|
- Which of the following statement(s) is /are true
Text Solution
|
- Conversion of esters to aldehydes can be accomplished by
Text Solution
|
- Consider the following electrode processes of a cell, Cl^- rightarro...
Text Solution
|
- Which of the following is true for spontaneous adsorption of H2 gas wi...
Text Solution
|
- Consider the single electrode process 4H^ + 4e^- = 2H2 catalyzed by pl...
Text Solution
|
- Which of the following elements has the lowest melting point?
Text Solution
|
- The number of complementary Hydrogen bond(s) between a guanine and cyt...
Text Solution
|
- Given Delta Hr ^@ for CO2(g) , CO(g) and H2O(g) are -393.5, -110.5 and...
Text Solution
|
- Which one of the following is the strongest acid?
Text Solution
|
- The species having pyramidal shape according to VESPR theory is
Text Solution
|
- The bonding in diborane (B2H6) can be described by
Text Solution
|
- The monomers of Buna -S rubber are
Text Solution
|
- Heating a mixture of Cu2O and Cu2S will give
Text Solution
|