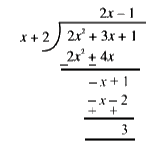Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise ENTRANCE QUESTION BANK (WBJEE)|1 VideosORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise ENTRANCE QUESTION BANK|97 VideosORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES
CHHAYA PUBLICATION|Exercise SLOVED NCERT EXERCISE|38 VideosMODEL QUESTIONS PAPER
CHHAYA PUBLICATION|Exercise SET III (SECTION II) (GROUP D )|12 VideosP-BLOCK ELEMENTS
CHHAYA PUBLICATION|Exercise PRACTICE SET 7 (ANSWER THE FOLLOWING QUESTIONS) : -|17 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-ORGANIC CHEMISTRY : BASIC PRINCIPLES AND TECHNIQUES -HIGHER ORDER THINKING SKILL (HOTS) QUESTION
- (1) Give structure and IUPAC name of an optically active alkane having...
Text Solution
|
- The following two isomers may be called diastereoisomers but not enant...
Text Solution
|
- P - nitrophenol is more acidic than phenol. Explain.
Text Solution
|
- P - nitroanilin is a weaker base than aniline. Explain.
Text Solution
|
- Dipole moment of vinyl chloride (CH(2) = CHCI) is less than the dipole...
Text Solution
|
- Arrange the following ions in order of increasing basicity and explain...
Text Solution
|
- Give example: (1) a non - nuclephilic anion (2) a planar carbocati...
Text Solution
|
- Explain the given basicity order in aqueous medium: (CH(3))(2)overse...
Text Solution
|
- Which of the two: O(2)NCH(2)CH(2)O^(Theta)orCH(3)CH(2)O^(Theta) is exp...
Text Solution
|
- CH(3)CI undergoes hydrolysis more easily than C(6)H(5)CI. Explain.
Text Solution
|
- Benzyl chloride participates in S(N)1 reaction even through it is a pr...
Text Solution
|
- Bond dissociation enthalpy of C(6)H(5)CH(2) - H bond is much less than...
Text Solution
|
- N,N,2,6 - Tetramethylaniline is more basic than N,N - dimethylaniline....
Text Solution
|
- Chloroform is more acidic than fluoroform. Explain.
Text Solution
|
- How can you separate benzoic acid and nitro benzene from their mixture...
Text Solution
|
- How will you separate benzyl alcohol (neutral) and phenol (acidic) fro...
Text Solution
|
- Why is impure glycerol purified by distillation under reduced pressure...
Text Solution
|
- Why is it necessary to use acetic acid and not sulphuric acid for acid...
Text Solution
|
- Preence of N is hydroxylamine hydrochloride cannot be detected by Lass...
Text Solution
|
- How can it be possible to detect the presence of nitrogen in hydrazin...
Text Solution
|