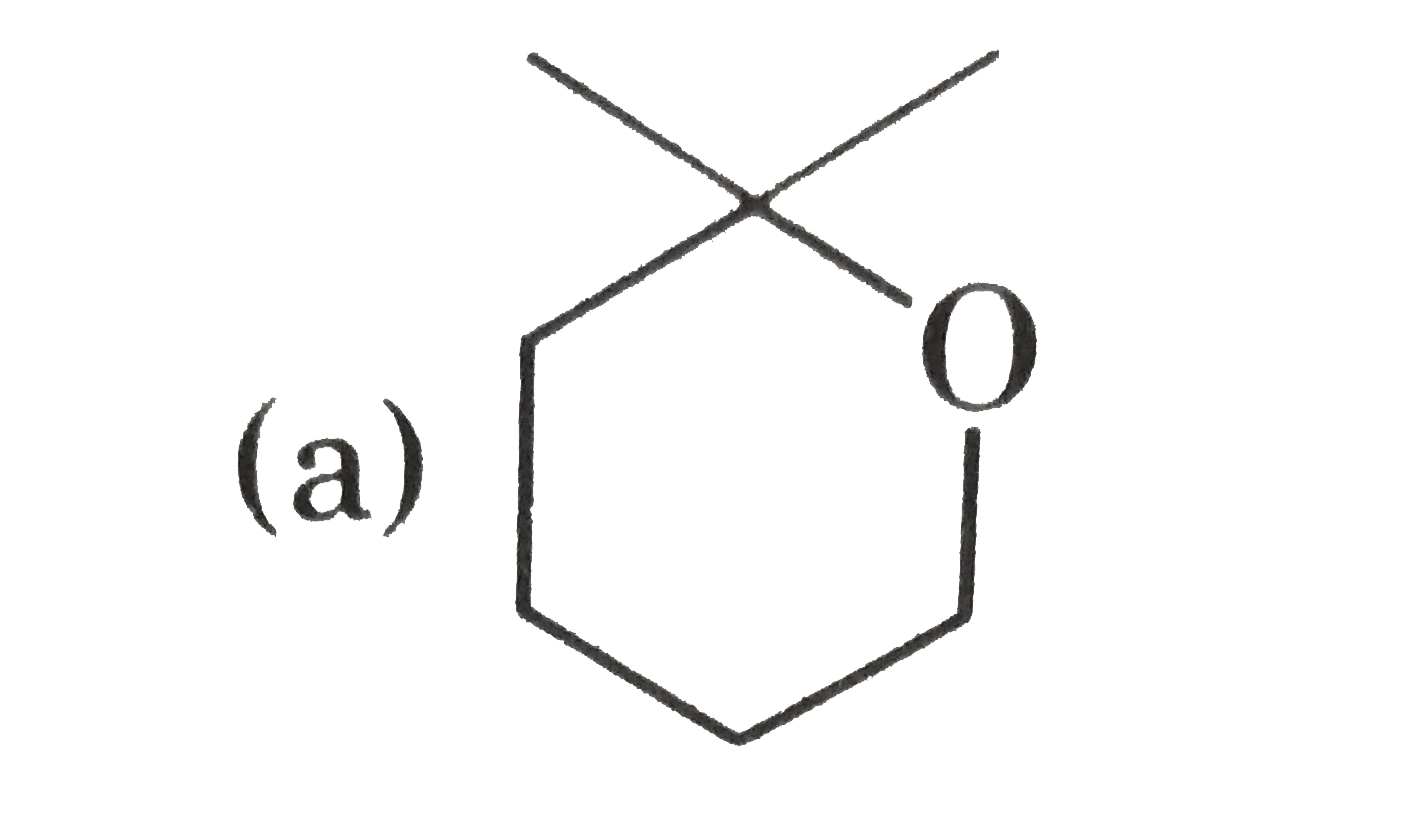
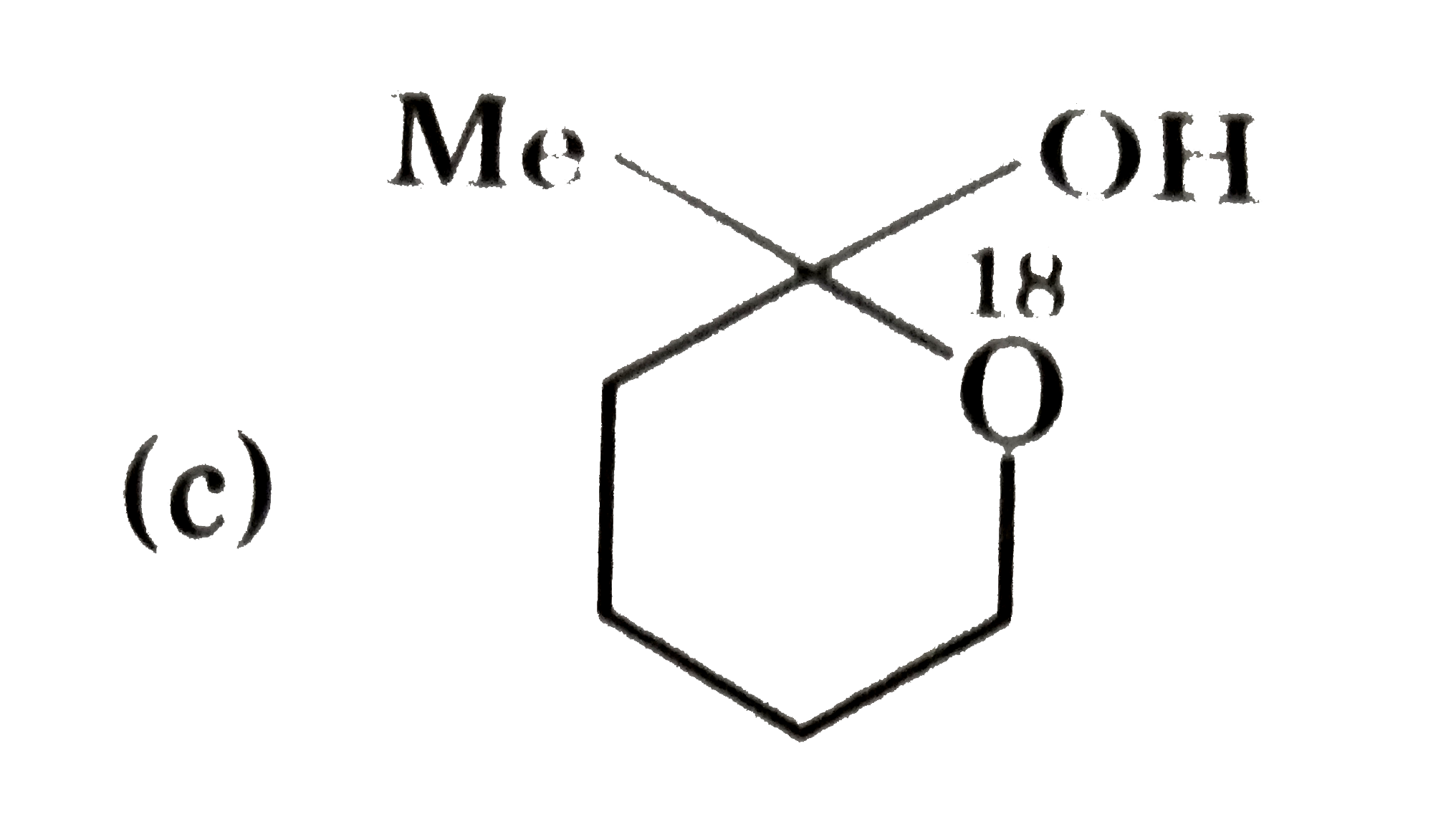
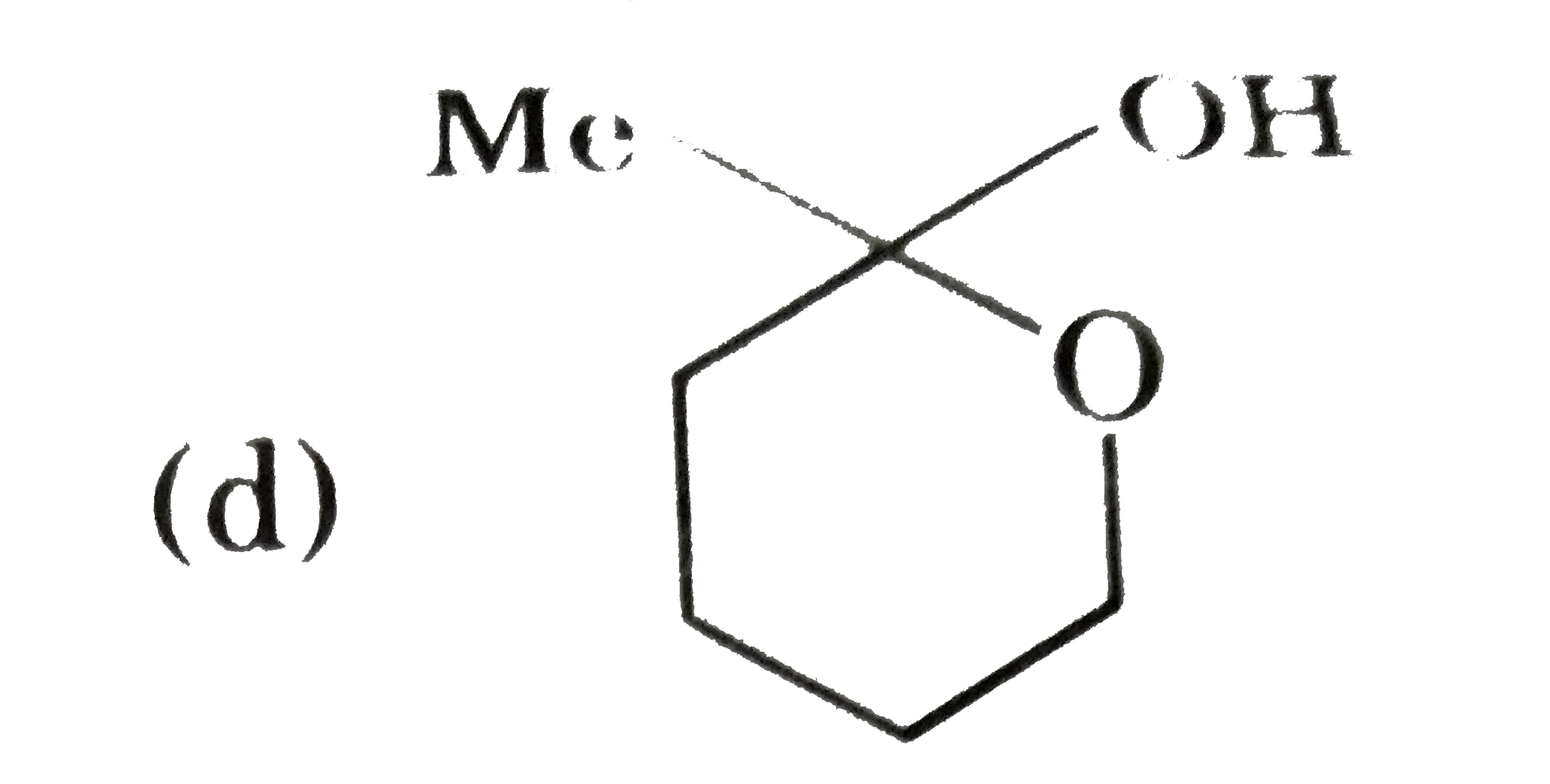
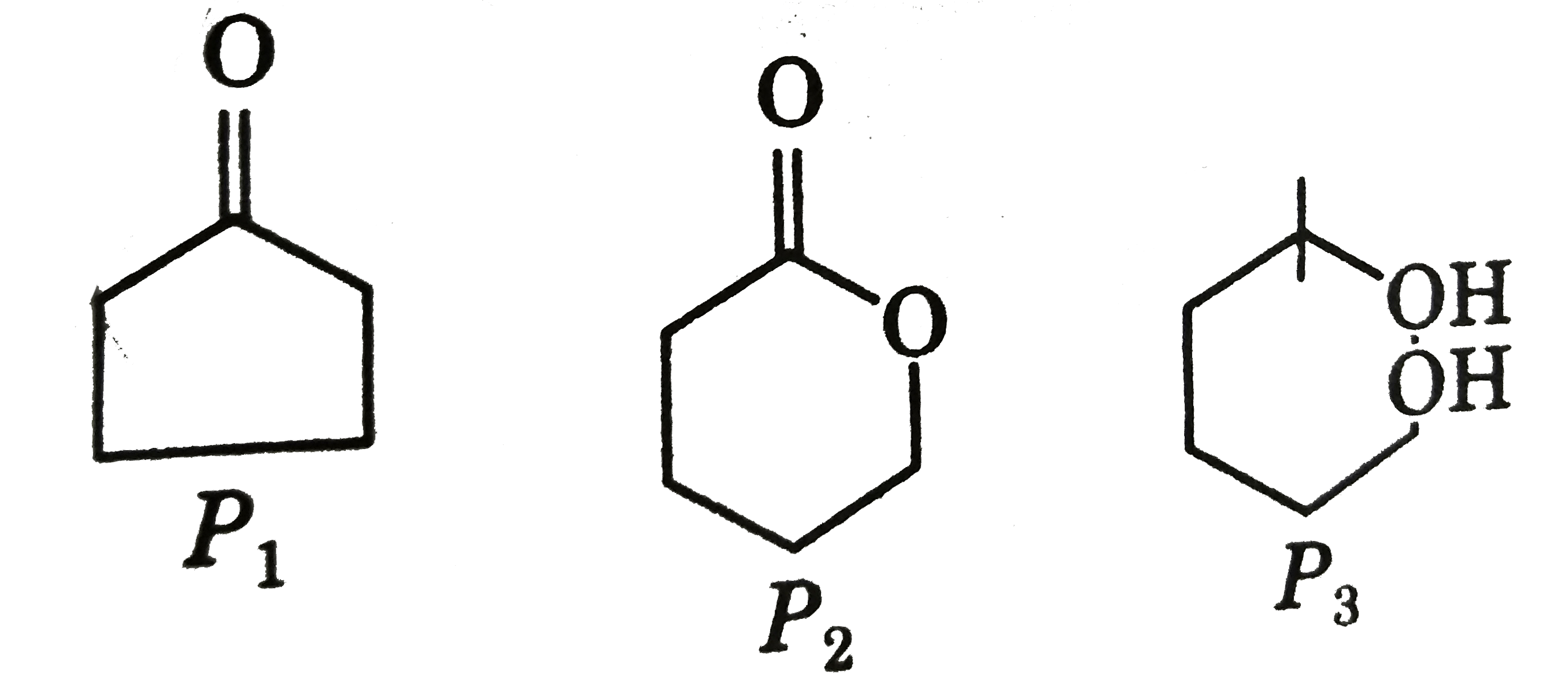
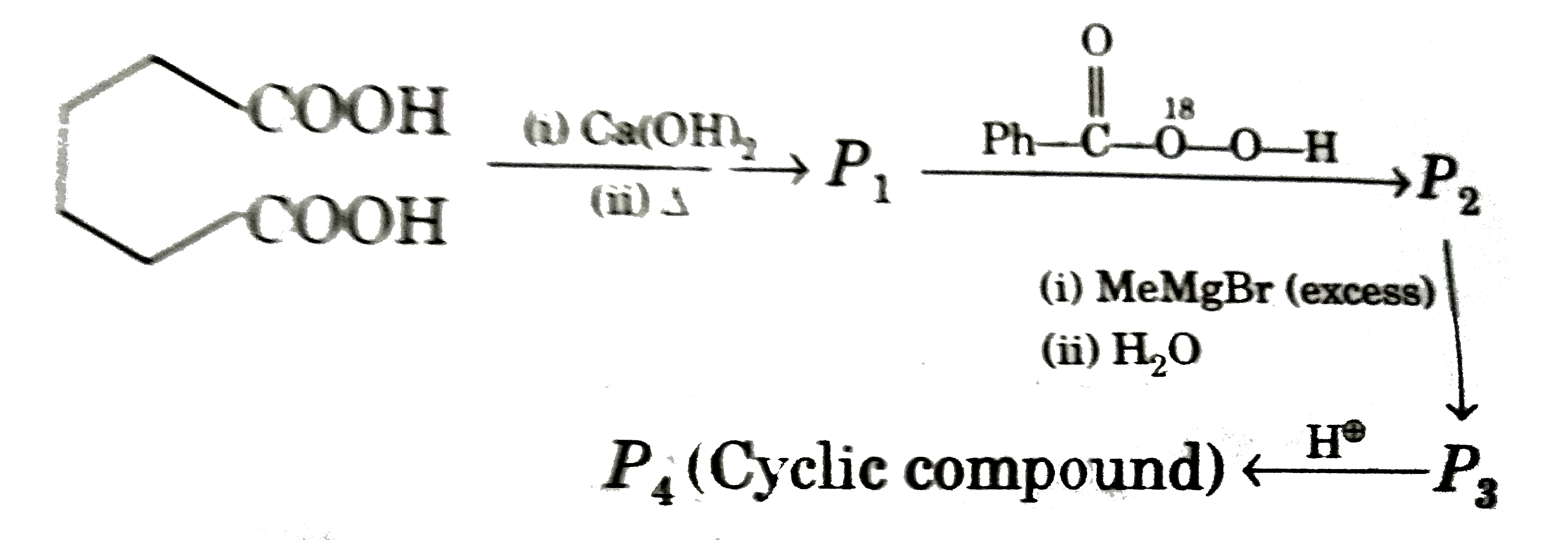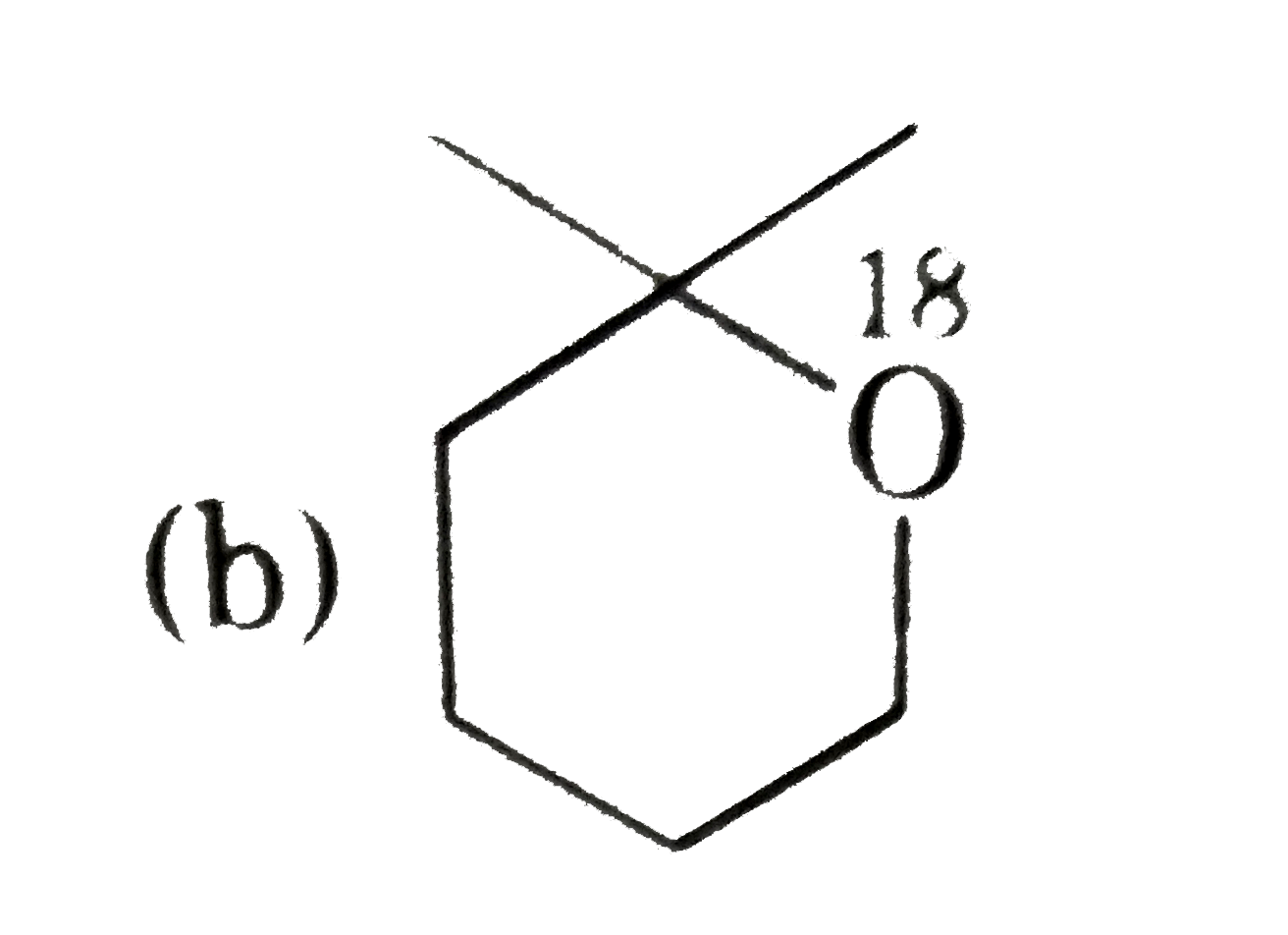A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDS
GRB PUBLICATION|Exercise REASONING TYPE|9 VideosCARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDS
GRB PUBLICATION|Exercise MULTIPLE OBJECTIVE TYPE|48 VideosBIOMOLECULES, POLYMERS, PRACTICAL ORGANIC CHEMISTRY AND CHEMISTRY IN DAILY LIFE
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|40 VideosCHEMICAL BONDING-I
GRB PUBLICATION|Exercise Subjective Type|120 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-CARBOXYLIC ACID AND DERIVATIVES, AMINES AND OTHER NITROGEN COMPOUNDS-Subjective type
- P(4) is :
Text Solution
|
- Write the number of CO(2) molecule : For example if all are releasi...
Text Solution
|
- Convert in exactly four steps. Select the regent from the given set ...
Text Solution
|
- Number of products formed.
Text Solution
|
- Number of compounds, which can evolve CO(2) on heating, are…….
Text Solution
|
- Number of reaction which gives ester as major product.
Text Solution
|
- Number of acylon product(s) formed during this reaction. Me-overset(...
Text Solution
|
- How many moles of CO(2) per mole will release on heating of following ...
Text Solution
|
- Formic acid can be detected with how many of the followings tests ? ...
Text Solution
|
- A compound with molecular formula C(6)H(14)O(4) does not give litmus t...
Text Solution
|
- A compound having molecular formula C(5)H(10)O(5) was reacted with exc...
Text Solution
|
- Et-O-overset(O)overset(|)C-O-Et overset((i)"z PhMgBr")underset((ii)H^(...
Text Solution
|
- PhCOOHunderset(Cl(2))overset(Red P(limited))to How many Cl atoms are...
Text Solution
|
- A compound with molecular formula C(8)H(18)O(4) does not give litmus t...
Text Solution
|
- Consider thew reaction given below :
Text Solution
|
- How many 1^(@) amine of the formula C(5)H(13)N can be used to resolve ...
Text Solution
|
- How amny amines are possible by C(4)H(11)N which form product having u...
Text Solution
|
- x= moles of KOH consumed is :
Text Solution
|
- Cl-CH(2)-overset(14)CH(2)-S-CH(2)-CH(3)overset(NH(3))to number of am...
Text Solution
|
- Number of moles of N(2) liberated by reaction of with NaNO(2)//HC...
Text Solution
|