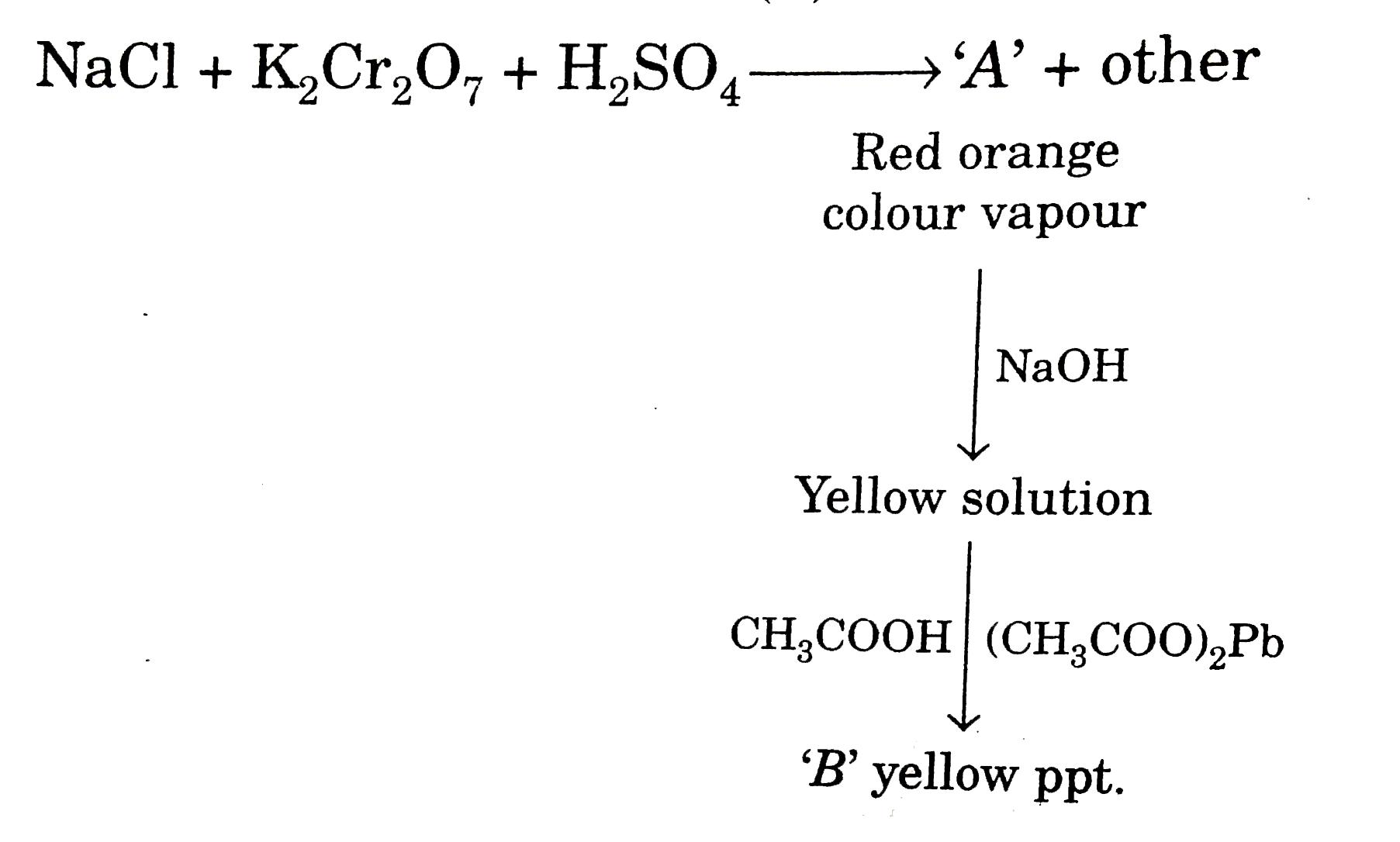A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise E.Precipitation/Redox Reacttions|48 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise F.Zero Group Cations|18 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise C.Class- A Subgroup-I-Acidic Radicals|50 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
GRB PUBLICATION-SALT ANALYSIS-D.Class-A Subgroup-II Acidic Radicals
- Nitrite (NO(2)^(-)) interferes in the 'ring-test' of nitrate (NO(3)^(-...
Text Solution
|
- Find the sum of number of sigma-bond in A,B and C.
Text Solution
|
- Choose the incorrect statement:
Text Solution
|
- The anion which does not produce diatomic gas on treatement with hot a...
Text Solution
|
- Which of the following reagents turns white precipitate of AgCl yellow...
Text Solution
|
- When a mixture of solid NaCl and solid K(2)Cr(2)O(7) is heated with co...
Text Solution
|
- AgCl dissolves in ammonia solution giving
Text Solution
|
- A mixture upon along adding conc. H(2)SO(4) gives deep red fumes. It m...
Text Solution
|
- A solution of a salt in concentrated sulphuric acid H(2)SO(4) acid pro...
Text Solution
|
- A colouless solution of a compound gives a precipitate with AgNO(3) so...
Text Solution
|
- When chlorine (Cl(2)) water in excess is added to a salt solution cont...
Text Solution
|
- An aqueous solution of salt containing an acidic radical X^(-) reacts ...
Text Solution
|
- When chlorine water is added to an aqueous solution of potassium halid...
Text Solution
|
- Nitrate is confirmed by ring test. The brown colour of the ring is du...
Text Solution
|
- Nitrates of all the metals except mercury and bismuth are:
Text Solution
|
- Identify the correct statement:
Text Solution
|
- Which of the following thermal decomposition yields a basic as well as...
Text Solution
|
- Choose the correct statement from the following:
Text Solution
|
- BO(3)^(3-) + underset("conc")(H(2)SO(4))overset(Delta)rarr underset("W...
Text Solution
|
- CaC(2)O(4)+AcOH overset(Na(2)CO(3))underset("Solution")rarr(X), then X...
Text Solution
|