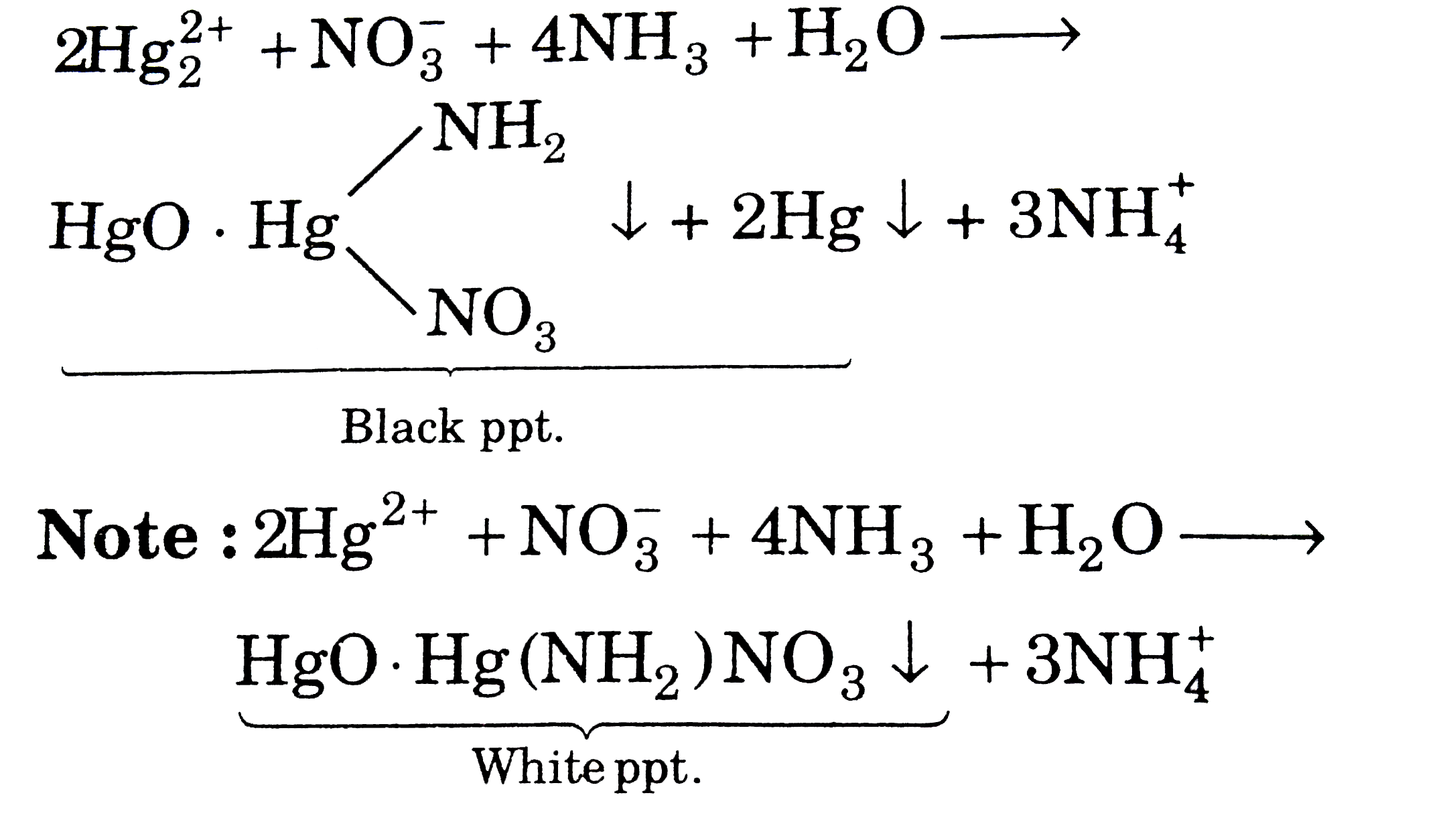A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise G: Ist Group Cations|20 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise H: IInd Group Cations|49 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise E.Precipitation/Redox Reacttions|48 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-F.Zero Group Cations
- A white crystalline solid (A) on boiling with caustic soda solution ga...
Text Solution
|
- The reagents, NH(4)CI and aqueous NH(3) will precipitate:
Text Solution
|
- In the precipitation of the iron group in qualitative analysis, ammoni...
Text Solution
|
- Which one of the following can be used in place of NH(4)Cl for the ide...
Text Solution
|
- Which one of the following statement is correct?
Text Solution
|
- Mark the correct statement:
Text Solution
|
- (X)overset(KOH)to (Y) (gas turns red litmus blue)+(Z)overset(Zn+KOH)to...
Text Solution
|
- When NH(4)OH is added in Hg(2)(NO(3))(2) solution, the ppt formed is:
Text Solution
|
- Calcium imide on hydrolysis will give gas (B) which on oxidation by be...
Text Solution
|
- When solid KOH is mixed with solid NH(4)CI a gas is produced. Which ga...
Text Solution
|
- Compound A on hydrolysis gives gaseous compound B and alkaline solutio...
Text Solution
|
- An element M forms insoluble hydroxide, which is soluble in both exces...
Text Solution
|
- Select the correct statement out of following:
Text Solution
|
- An aqueous solution of compound 'A' gives white precipitate with 2M HC...
Text Solution
|
- 'A' +NaOH rarr 'B' ("gas") +'C' overset(Zn)rarr 'B' underset(("gas...
Text Solution
|
- Nessler's reagent is
Text Solution
|
- Ammonia/ammonium ion gives yellow precipitate with:
Text Solution
|
- Ammonium salts on heating with slaked lime liberates a colourless gas ...
Text Solution
|