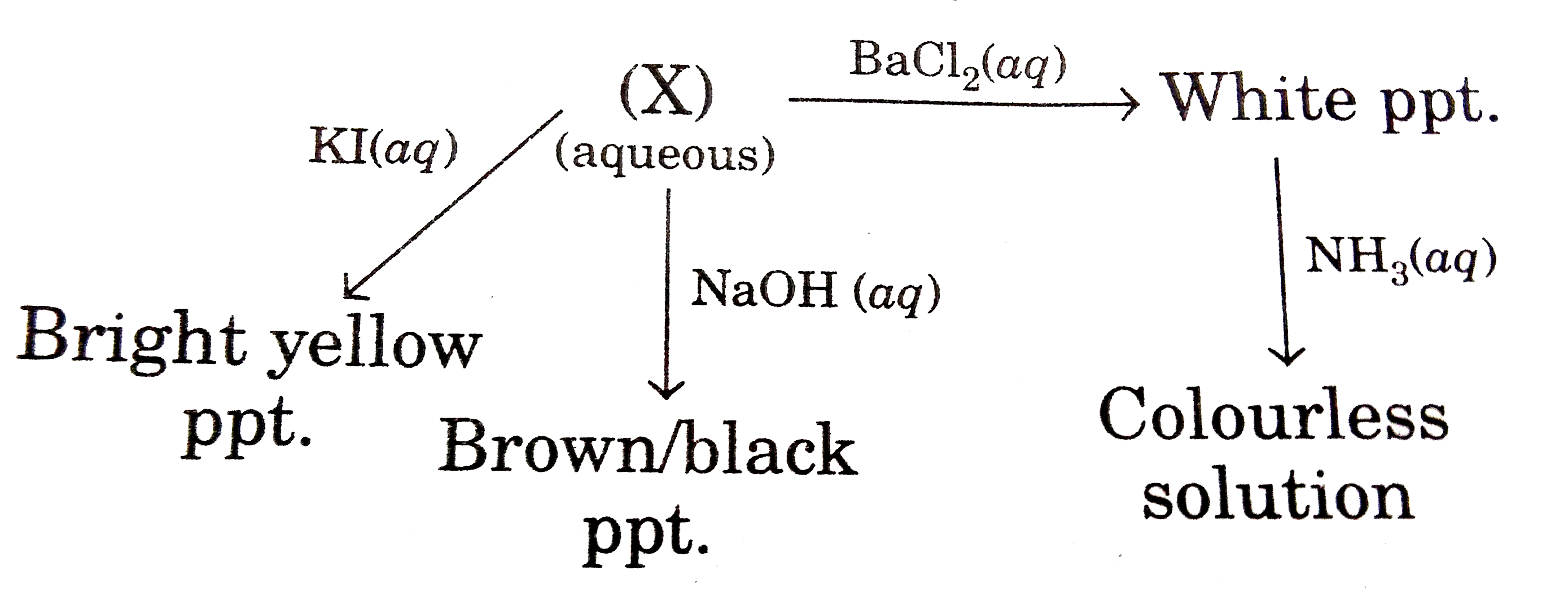A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise H: IInd Group Cations|49 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise I. IIIrd Group Cations|36 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise F.Zero Group Cations|18 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-G: Ist Group Cations
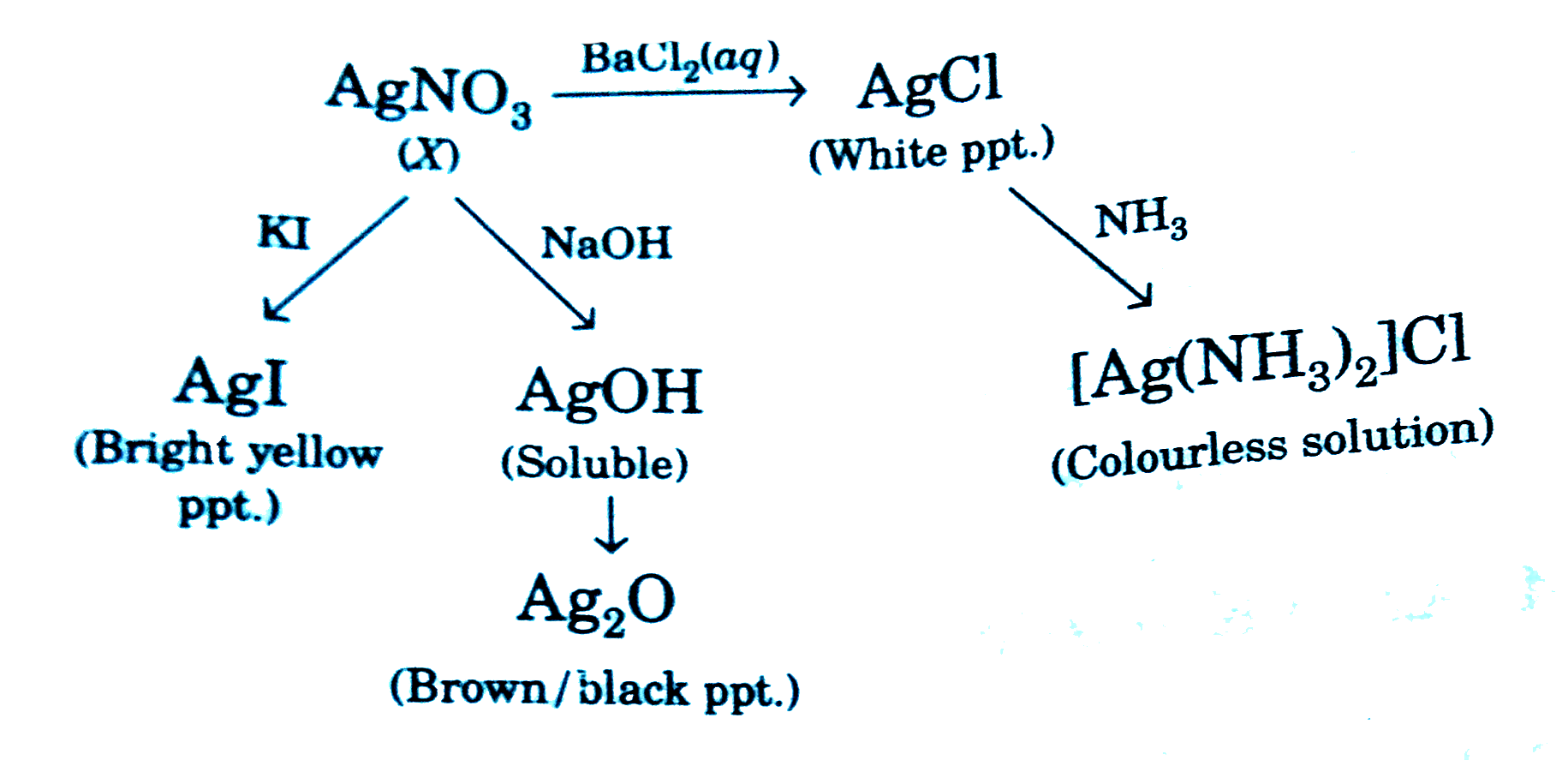
- A compound (X) reacts in the following ways: The compound (X) is...
Text Solution
|
- To a solution of a substance, gradual addition of ammonium hydroxide r...
Text Solution
|
- Identity the compound which turns black with ammonia solution.
Text Solution
|
- A white crystalline substance dissolves in water.On passing H(2)S in t...
Text Solution
|
- The composition of golden spangles is
Text Solution
|
- In which of the following solvents, AgBr will have the highest solubil...
Text Solution
|
- A metal nitrate solution reacts with dilute hydrochloric acid to give ...
Text Solution
|
- A metal nitrate solution does not give white precipitate with concentr...
Text Solution
|
- How can 0.1g samples of the two white solids, lead (II) chloride and s...
Text Solution
|
- A sample of sparingly soluble PbI(2)(s) containing radioactive I-133 i...
Text Solution
|
- Which solid does not react with a small amount of 3 M HNO(3)?
Text Solution
|
- A 2.0mL sample of a colourless solution, when treated with a few drops...
Text Solution
|
- Which of the following substance cannot be obtained when Hg(2)F(2) is ...
Text Solution
|
- A metal nitrate reacts with Kl solution to give yellow precipitate whi...
Text Solution
|
- Three separate samples of a solution of a single salt gave these resul...
Text Solution
|
- Cu^(2+) and Ag^(+) are both present in the same solution.To precipitat...
Text Solution
|
- Consider the following observation: M(n+) + HCl (dilute) to white pr...
Text Solution
|
- A white crystalline substance dissolves in water.On passing H(2)S in t...
Text Solution
|
- A red solid is insoluble in water. However, it becomes soluble if some...
Text Solution
|
- Mercurous ion is represent as
Text Solution
|