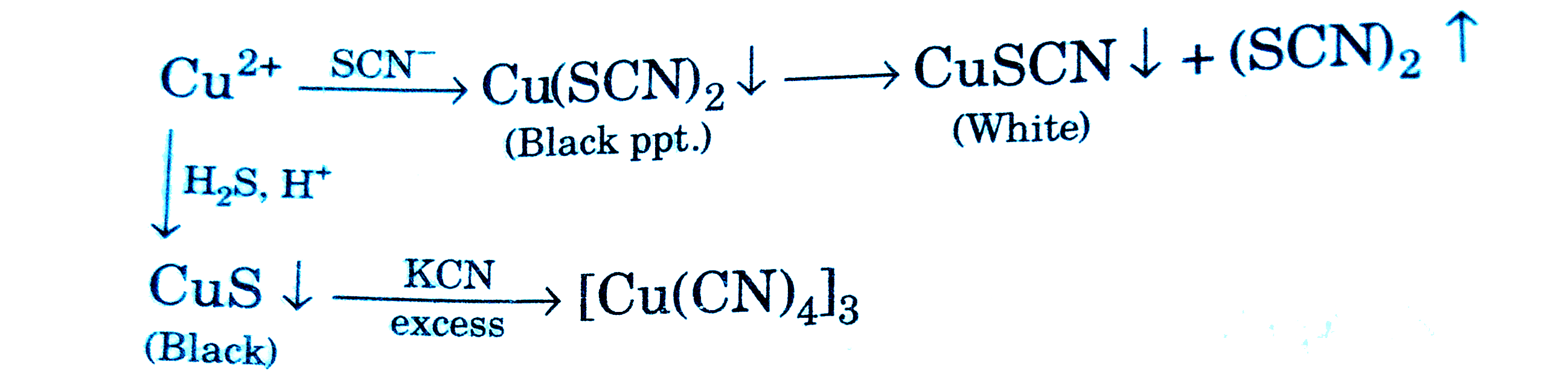A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SALT ANALYSIS
GRB PUBLICATION|Exercise I. IIIrd Group Cations|36 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise J: IVth Group Cations|24 VideosSALT ANALYSIS
GRB PUBLICATION|Exercise G: Ist Group Cations|20 VideosS BLOCK ELEMENTS
GRB PUBLICATION|Exercise SUBJECTIVE TYPE|9 VideosTHERMODYNAMICS
GRB PUBLICATION|Exercise All Questions|704 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-SALT ANALYSIS-H: IInd Group Cations
- Which of the following is not precipitated as sulphide by passing H(2)...
Text Solution
|
- Which of the following metal cation is reduced from its higher oxidati...
Text Solution
|
- A coloured solution of an inorganic salt reacts with potassium thiocya...
Text Solution
|
- Which of the following reagents gives a yellow precipitate with a hot ...
Text Solution
|
- Select the incorrect statement.
Text Solution
|
- Aq. Suspension of a yellow substance (A) overset(KI)underset("conc.sol...
Text Solution
|
- Brown ppt.(A) dissolve in HNO(3) gives (B) which gives white ppt. (C) ...
Text Solution
|
- Colourless solutions of the following four salts are placed separately...
Text Solution
|
- Identify the hydrated blue salt which when treated with KI solution, a...
Text Solution
|
- Which of the following is soluble is yellow ammonium sulphide?
Text Solution
|
- A solution of concentrated aqueous ammonia is added dropwise to 1mL of...
Text Solution
|
- A colourless solution is known to contain one of these ions. Which ion...
Text Solution
|
- What are formed products, when aqueous solution of CuCl(2) and (NH(4))...
Text Solution
|
- What products result when equal volumes of equimolar aqueous solutions...
Text Solution
|
- Which solution produces a black precipitate when added to an aqueous c...
Text Solution
|
- Which cation forms an insoluble chloride and an insoluble sulphide?
Text Solution
|
- Select reaction in which oxidation number of copper is not changed in ...
Text Solution
|
- Which compound is formed when excess KCN is added to aqueous solution ...
Text Solution
|
- Consider the following statements: Statement-1: Cu^(2+) ions are red...
Text Solution
|
- Chocolate brown precipitate is formed with:
Text Solution
|